Steroidogenic cytochrome P450 17A1 structure and function
- PMID: 33781841
- PMCID: PMC8087655
- DOI: 10.1016/j.mce.2021.111261
Steroidogenic cytochrome P450 17A1 structure and function
Abstract
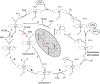
Cytochrome P450 17A1 (CYP17A1) is a critical steroidogenic enzyme, essential for producing glucocorticoids and sex hormones. This review discusses the complex activity of CYP17A1, looking at its role in both the classical and backdoor steroidogenic pathways and the complex chemistry it carries out to perform both a hydroxylation reaction and a carbon-carbon cleavage, or lyase reaction. Functional and structural investigations have informed our knowledge of these two reactions. This review focuses on a few specific aspects of this discussion: the identities of reaction intermediates, the coordination of hydroxylation and lyase reactions, the effects of cytochrome b5, and conformational selection. These discussions improve understanding of CYP17A1 in a physiological setting, where CYP17A1 is implicated in a variety of steroidogenic diseases. This information can be used to improve ways in which CYP17A1 can be effectively modulated to treat diseases such as prostate and breast cancer, Cushing's syndrome, and glioblastoma.
Keywords: Cancer; Crystallography; Cushing's syndrome; Cytochrome P450 17A1; Mechanism; Prostate.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
DECLARATION OF COMPETING INTERESTS
The authors have no conflict of interests, except that EES is co-inventor on a patent for an abiraterone analog discussed in section 4.
Figures

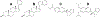
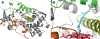

Similar articles
-
Substrate-modulated cytochrome P450 17A1 and cytochrome b5 interactions revealed by NMR.J Biol Chem. 2013 Jun 7;288(23):17008-17018. doi: 10.1074/jbc.M113.468926. Epub 2013 Apr 25. J Biol Chem. 2013. PMID: 23620596 Free PMC article.
-
Structural insights into the function of steroidogenic cytochrome P450 17A1.Mol Cell Endocrinol. 2017 Feb 5;441:68-75. doi: 10.1016/j.mce.2016.08.035. Epub 2016 Aug 24. Mol Cell Endocrinol. 2017. PMID: 27566228 Free PMC article. Review.
-
Role of cytochrome b5 in the modulation of the enzymatic activities of cytochrome P450 17α-hydroxylase/17,20-lyase (P450 17A1).J Steroid Biochem Mol Biol. 2017 Jun;170:2-18. doi: 10.1016/j.jsbmb.2016.02.033. Epub 2016 Mar 11. J Steroid Biochem Mol Biol. 2017. PMID: 26976652 Review.
-
Human cytochrome P450 17A1 conformational selection: modulation by ligand and cytochrome b5.J Biol Chem. 2014 May 16;289(20):14310-20. doi: 10.1074/jbc.M114.560144. Epub 2014 Mar 26. J Biol Chem. 2014. PMID: 24671419 Free PMC article.
-
Structures of human steroidogenic cytochrome P450 17A1 with substrates.J Biol Chem. 2014 Nov 21;289(47):32952-64. doi: 10.1074/jbc.M114.610998. Epub 2014 Oct 9. J Biol Chem. 2014. PMID: 25301938 Free PMC article.
Cited by
-
Effects of Dietary Cholesterol Regulation on Spermatogenesis of Gobiocypris rarus Rare Minnow.Int J Mol Sci. 2023 Apr 19;24(8):7492. doi: 10.3390/ijms24087492. Int J Mol Sci. 2023. PMID: 37108655 Free PMC article.
-
Non-steroidal CYP17A1 Inhibitors: Discovery and Assessment.J Med Chem. 2023 May 25;66(10):6542-6566. doi: 10.1021/acs.jmedchem.3c00442. Epub 2023 May 16. J Med Chem. 2023. PMID: 37191389 Free PMC article. Review.
-
Decoding the Role of CYP450 Enzymes in Metabolism and Disease: A Comprehensive Review.Biomedicines. 2024 Jul 2;12(7):1467. doi: 10.3390/biomedicines12071467. Biomedicines. 2024. PMID: 39062040 Free PMC article. Review.
-
Hydroxylation and lyase reactions of steroids catalyzed by mouse cytochrome P450 17A1 (Cyp17a1).J Inorg Biochem. 2023 Mar;240:112085. doi: 10.1016/j.jinorgbio.2022.112085. Epub 2023 Jan 10. J Inorg Biochem. 2023. PMID: 36640554 Free PMC article.
-
Metabolites of Cannabigerol Generated by Human Cytochrome P450s Are Bioactive.Biochemistry. 2022 Nov 1;61(21):2398-2408. doi: 10.1021/acs.biochem.2c00383. Epub 2022 Oct 12. Biochemistry. 2022. PMID: 36223199 Free PMC article.
References
-
- Akhtar M, Corina D, Miller S, Shyadehi AZ and Wright JN, 1994. Mechanism of the acyl-carbon cleavage and related reactions catalyzed by multifunctional P-450s: Studies on cytochrome P-450(17)alpha, Biochemistry. 33, 4410–8. - PubMed
-
- Akhtar M, Wright JN and Lee-Robichaud P, 2011. A review of mechanistic studies on aromatase (CYP19) and 17alpha-hydroxylase-17,20-lyase (CYP17), J Steroid Biochem Mol Biol. 125, 2–12. - PubMed
-
- Amicis FD, Thirugnansampanthan J, Cui Y, Selever J, Beyer A, Parra I, Weigel NL, Herynk MH, Tsimelzon A, Lewis MT, Chamness GC, Hilsenbeck SG, Andò S and Fuqua SAW, 2010. Androgen receptor overexpression induces tamoxifen resistance in human breast cancer cells, Breast Cancer Res Treat. 121, 1–11. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

