Intramyocardial Transplantation of Human iPS Cell-Derived Cardiac Spheroids Improves Cardiac Function in Heart Failure Animals
- PMID: 33778211
- PMCID: PMC7987543
- DOI: 10.1016/j.jacbts.2020.11.017
Intramyocardial Transplantation of Human iPS Cell-Derived Cardiac Spheroids Improves Cardiac Function in Heart Failure Animals
Abstract
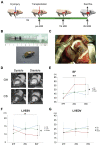
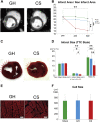
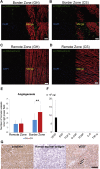
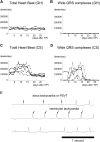
The severe shortage of donor hearts hampered the cardiac transplantation to patients with advanced heart failure. Therefore, cardiac regenerative therapies are eagerly awaited as a substitution. Human induced pluripotent stem cells (hiPSCs) are realistic cell source for regenerative cardiomyocytes. The hiPSC-derived cardiomyocytes are highly expected to help the recovery of heart. Avoidance of teratoma formation and large-scale culture of cardiomyocytes are definitely necessary for clinical setting. The combination of pure cardiac spheroids and gelatin hydrogel succeeded to recover reduced ejection fraction. The feasible transplantation strategy including transplantation device for regenerative cardiomyocytes are established in this study.
Keywords: CM, cardiomyocyte; CMR, cardiac magnetic resonance; CS, cardiac spheroid; ECG, electrocardiogram; EF, ejection fraction; FAC, fractional area change; GH, gelatin hydrogel; HF, heart failure; LV, left ventricular; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; VEGF, vascular endothelial growth factor; cardiac spheroids; cardiomyocyte; cell transplantation; dp/dtmax, maximum rate of left ventricular pressure rise; hPSC, human pluripotent stem cell; heart failure; hiPSC, human induced pluripotent stem cell; human iPS cells; sCM, single cardiomyocyte.
© 2021 The Authors.
Conflict of interest statement
This work was supported by the Highway Program for Realization of Regenerative Medicine (17bm054006h0007 [to Dr. Fukuda]) and the Research Project for Practical Applications of Regenerative Medicine (17bk010462h0001 [to Dr. Fukuda]) from the Japan Agency for Medical Research and Development, and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (nos. 15K09098 [to Dr. Kanazawa], 16K09507 [to Dr. Fujita], 19H03660 [to Dr. Fujita], 17H05067 [to Dr. Tohyama], 18K15903 [to Dr. Nakajima]). Drs. Kanazawa, Tohyama, Fukuda, and Fujita have patents related to this work. Drs. Tohyama, Shimizu, Kanazawa, Fukuda, and Fujita own equity in Heartseed, Inc. Dr. Tohyama is an advisor of Heartseed, Inc. Dr. Fukuda is a co-founder and CEO of Heartseed, Inc.; and receives a salary from Heartseed, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures
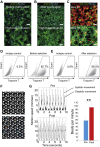
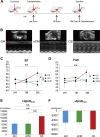

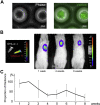
Comment in
-
Balls to the Wall: Human Pluripotent Cell-Derived Cardiac Muscle Spheres Enhance Preclinical Heart Repair.JACC Basic Transl Sci. 2021 Mar 22;6(3):255-256. doi: 10.1016/j.jacbts.2020.12.011. eCollection 2021 Mar. JACC Basic Transl Sci. 2021. PMID: 33779650 Free PMC article. No abstract available.
Similar articles
-
Therapeutic potential of clinical-grade human induced pluripotent stem cell-derived cardiac tissues.JTCVS Open. 2021 Oct 1;8:359-374. doi: 10.1016/j.xjon.2021.09.038. eCollection 2021 Dec. JTCVS Open. 2021. PMID: 36004071 Free PMC article.
-
Development of a transplant injection device for optimal distribution and retention of human induced pluripotent stem cell‒derived cardiomyocytes.J Heart Lung Transplant. 2019 Feb;38(2):203-214. doi: 10.1016/j.healun.2018.11.002. Epub 2018 Nov 15. J Heart Lung Transplant. 2019. PMID: 30691596
-
Spheroids of cardiomyocytes derived from human-induced pluripotent stem cells improve recovery from myocardial injury in mice.Am J Physiol Heart Circ Physiol. 2018 Aug 1;315(2):H327-H339. doi: 10.1152/ajpheart.00688.2017. Epub 2018 Apr 6. Am J Physiol Heart Circ Physiol. 2018. PMID: 29631371 Free PMC article.
-
Development of Cardiac Regenerative Medicine Using Human iPS Cell-derived Cardiomyocytes.Keio J Med. 2021 Sep 25;70(3):53-59. doi: 10.2302/kjm.2020-0009-IR. Epub 2020 Aug 22. Keio J Med. 2021. PMID: 32830153 Review.
-
Toward the realization of cardiac regenerative medicine using pluripotent stem cells.Inflamm Regen. 2020 Jan 13;40:1. doi: 10.1186/s41232-019-0110-4. eCollection 2020. Inflamm Regen. 2020. PMID: 31938077 Free PMC article. Review.
Cited by
-
Challenges and perspectives of heart repair with pluripotent stem cell-derived cardiomyocytes.Nat Cardiovasc Res. 2024 May;3(5):515-524. doi: 10.1038/s44161-024-00472-6. Epub 2024 May 14. Nat Cardiovasc Res. 2024. PMID: 39195938 Review.
-
Recognizing the Differentiation Degree of Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium Cells Using Machine Learning and Deep Learning-Based Approaches.Cells. 2023 Jan 4;12(2):211. doi: 10.3390/cells12020211. Cells. 2023. PMID: 36672144 Free PMC article.
-
Balls to the Wall: Human Pluripotent Cell-Derived Cardiac Muscle Spheres Enhance Preclinical Heart Repair.JACC Basic Transl Sci. 2021 Mar 22;6(3):255-256. doi: 10.1016/j.jacbts.2020.12.011. eCollection 2021 Mar. JACC Basic Transl Sci. 2021. PMID: 33779650 Free PMC article. No abstract available.
-
Engineered Tissue for Cardiac Regeneration: Current Status and Future Perspectives.Bioengineering (Basel). 2022 Oct 22;9(11):605. doi: 10.3390/bioengineering9110605. Bioengineering (Basel). 2022. PMID: 36354516 Free PMC article. Review.
-
Scalable production of homogeneous cardiac organoids derived from human pluripotent stem cells.Cell Rep Methods. 2023 Dec 18;3(12):100666. doi: 10.1016/j.crmeth.2023.100666. Cell Rep Methods. 2023. PMID: 38113855 Free PMC article.
References
-
- Ponikowski P., Anker S.D., AlHabib K.F. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1:4–25. - PubMed
-
- Ponikowski P., Voors A.A., Anker S.D. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. - PubMed
-
- Khush K.K., Cherikh W.S., Chambers D.C. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth Adult Heart Transplantation Report-2018; Focus Theme: Multiorgan Transplantation. J Heart Lung Transplant. 2018;37:1155–1168. - PubMed
-
- Behfar A., Crespo-Diaz R., Terzic A., Gersh B.J. Cell therapy for cardiac repair--lessons from clinical trials. Nat Rev Cardiol. 2014;11:232–246. - PubMed
-
- Takahashi K., Tanabe K., Ohnuki M. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

