Pemafibrate Pretreatment Attenuates Apoptosis and Autophagy during Hepatic Ischemia-Reperfusion Injury by Modulating JAK2/STAT3 β/PPAR α Pathway
- PMID: 33777128
- PMCID: PMC7972847
- DOI: 10.1155/2021/6632137
Pemafibrate Pretreatment Attenuates Apoptosis and Autophagy during Hepatic Ischemia-Reperfusion Injury by Modulating JAK2/STAT3 β/PPAR α Pathway
Abstract
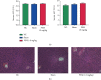
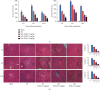
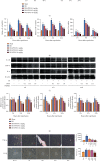
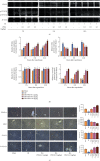
Hepatic ischemia-reperfusion injury (HIRI) is a common phenomenon in liver transplantation and liver surgery. This article is aimed at clarifying the role of pemafibrate in HIRI through JAK2/STAT3β/PPARα. In the experiment, we divided Balb/c into seven groups, namely, normal control (NC), Sham, PEM (1.0 mg/kg), IRI, IRI + PEM (0.1 mg/kg), IRI + PEM (0.5 mg/kg), and IRI + PEM (1.0 mg/kg). We used biochemical assay, histopathological evaluation, immunohistochemistry, RT-PCR and qRT-PCR, ELISA analysis, and other methods to determine the level of serum AST, ALT, IL-1β, and TNF-α in the liver at three time points (2 h, 8 h, and 24 h) after reperfusion of apoptosis factor, autophagy factor, and the JAK2/STAT3/PPARα content in tissues. Our experiment results showed that the pemafibrate can effectively reduce the level of hepatic IR injury. In addition, pemafibrate has anti-inflammatory, antiapoptotic, and antiautophagy effects, which are mediated by the JAK2/STAT3β/PPARα pathway.
Copyright © 2021 Ziqi Cheng and Chuanyong Guo.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Effect of sevoflurane on hepatic ischemia-reperfusion injury in rats via JAK2-STAT3 pathway.Eur Rev Med Pharmacol Sci. 2019 Feb;23(3):1350-1356. doi: 10.26355/eurrev_201902_17030. Eur Rev Med Pharmacol Sci. 2019. PMID: 30779103
-
Pemafibrate suppresses oxidative stress and apoptosis under cardiomyocyte ischemia-reperfusion injury in type 1 diabetes mellitus.Exp Ther Med. 2021 Apr;21(4):331. doi: 10.3892/etm.2021.9762. Epub 2021 Feb 8. Exp Ther Med. 2021. PMID: 33732304 Free PMC article.
-
Cafestol preconditioning attenuates apoptosis and autophagy during hepatic ischemia-reperfusion injury by inhibiting ERK/PPARγ pathway.Int Immunopharmacol. 2020 Jul;84:106529. doi: 10.1016/j.intimp.2020.106529. Epub 2020 Apr 27. Int Immunopharmacol. 2020. PMID: 32344356
-
The Protective Effect of Magnesium Lithospermate B on Hepatic Ischemia/Reperfusion via Inhibiting the Jak2/Stat3 Signaling Pathway.Front Pharmacol. 2019 May 31;10:620. doi: 10.3389/fphar.2019.00620. eCollection 2019. Front Pharmacol. 2019. PMID: 31231218 Free PMC article.
-
Autophagy in hepatic ischemia-reperfusion injury.Cell Death Discov. 2023 Apr 5;9(1):115. doi: 10.1038/s41420-023-01387-0. Cell Death Discov. 2023. PMID: 37019879 Free PMC article. Review.
Cited by
-
Phenolic Acids from Fructus Chebulae Immaturus Alleviate Intestinal Ischemia-Reperfusion Injury in Mice through the PPARα/NF-κB Pathway.Molecules. 2022 Aug 16;27(16):5227. doi: 10.3390/molecules27165227. Molecules. 2022. PMID: 36014464 Free PMC article.
-
Selective activation of PPARα maintains thermogenic capacity of beige adipocytes.iScience. 2023 Jun 19;26(7):107143. doi: 10.1016/j.isci.2023.107143. eCollection 2023 Jul 21. iScience. 2023. PMID: 37456852 Free PMC article.
-
Luteolin Pretreatment Attenuates Hepatic Ischemia-Reperfusion Injury in Mice by Inhibiting Inflammation, Autophagy, and Apoptosis via the ERK/PPARα Pathway.PPAR Res. 2022 Aug 3;2022:8161946. doi: 10.1155/2022/8161946. eCollection 2022. PPAR Res. 2022. PMID: 35966821 Free PMC article.
-
The research development of STAT3 in hepatic ischemia-reperfusion injury.Front Immunol. 2023 Jan 24;14:1066222. doi: 10.3389/fimmu.2023.1066222. eCollection 2023. Front Immunol. 2023. PMID: 36761734 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

