Viruses and Endogenous Retroviruses as Roots for Neuroinflammation and Neurodegenerative Diseases
- PMID: 33776642
- PMCID: PMC7994506
- DOI: 10.3389/fnins.2021.648629
Viruses and Endogenous Retroviruses as Roots for Neuroinflammation and Neurodegenerative Diseases
Abstract
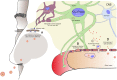
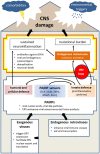
Many neurodegenerative diseases are associated with chronic inflammation in the brain and periphery giving rise to a continuous imbalance of immune processes. Next to inflammation markers, activation of transposable elements, including long intrespersed nuclear elements (LINE) elements and endogenous retroviruses (ERVs), has been identified during neurodegenerative disease progression and even correlated with the clinical severity of the disease. ERVs are remnants of viral infections in the human genome acquired during evolution. Upon activation, they produce transcripts and the phylogenetically youngest ones are still able to produce viral-like particles. In addition, ERVs can bind transcription factors and modulate immune response. Being between own and foreign, ERVs are reviewed in the context of viral infections of the central nervous system, in aging and neurodegenerative diseases. Moreover, this review tests the hypothesis that viral infection may be a trigger at the onset of neuroinflammation and that ERVs sustain the inflammatory imbalance by summarizing existing data of neurodegenerative diseases associated with viruses and/or ERVs.
Keywords: HERV; LINE; neurodegeneration; neuroinflammation; virus.
Copyright © 2021 Römer.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The Molecular Link Between TDP-43, Endogenous Retroviruses and Inflammatory Neurodegeneration in Amyotrophic Lateral Sclerosis: a Potential Target for Triumeq, an Antiretroviral Therapy.Mol Neurobiol. 2023 Nov;60(11):6330-6345. doi: 10.1007/s12035-023-03472-y. Epub 2023 Jul 14. Mol Neurobiol. 2023. PMID: 37450244 Free PMC article. Review.
-
Activation of the innate immune response by endogenous retroviruses.J Gen Virol. 2015 Jun;96(Pt 6):1207-1218. doi: 10.1099/jgv.0.000017. J Gen Virol. 2015. PMID: 26068187 Review.
-
Human Endogenous Retrovirus Type K (HERV-K) Particles Package and Transmit HERV-K-Related Sequences.J Virol. 2015 Jul;89(14):7187-201. doi: 10.1128/JVI.00544-15. Epub 2015 Apr 29. J Virol. 2015. PMID: 25926654 Free PMC article.
-
Endogenous retroviruses and MS: using ERVs as disease markers.Int MS J. 2003 Apr;10(1):22-8. Int MS J. 2003. PMID: 12906767 Review.
-
Genome-Wide Characterization of Zebrafish Endogenous Retroviruses Reveals Unexpected Diversity in Genetic Organizations and Functional Potentials.Microbiol Spectr. 2021 Dec 22;9(3):e0225421. doi: 10.1128/spectrum.02254-21. Epub 2021 Dec 15. Microbiol Spectr. 2021. PMID: 34908463 Free PMC article.
Cited by
-
Mycobacterium paratuberculosis: A HERV Turn-On for Autoimmunity, Neurodegeneration, and Cancer?Microorganisms. 2024 Sep 13;12(9):1890. doi: 10.3390/microorganisms12091890. Microorganisms. 2024. PMID: 39338563 Free PMC article. Review.
-
Arc protein, a remnant of ancient retrovirus, forms virus-like particles, which are abundantly generated by neurons during epileptic seizures, and affects epileptic susceptibility in rodent models.Front Neurol. 2023 Jul 7;14:1201104. doi: 10.3389/fneur.2023.1201104. eCollection 2023. Front Neurol. 2023. PMID: 37483450 Free PMC article. Review.
-
Systems Biology to Understand and Regulate Human Retroviral Proinflammatory Response.Front Immunol. 2021 Nov 16;12:736349. doi: 10.3389/fimmu.2021.736349. eCollection 2021. Front Immunol. 2021. PMID: 34867957 Free PMC article. Review.
-
The Influence of Virus Infection on Microglia and Accelerated Brain Aging.Cells. 2021 Jul 20;10(7):1836. doi: 10.3390/cells10071836. Cells. 2021. PMID: 34360004 Free PMC article. Review.
-
CancerHERVdb: Human Endogenous Retrovirus (HERV) Expression Database for Human Cancer Accelerates Studies of the Retrovirome and Predictions for HERV-Based Therapies.J Virol. 2023 Jun 29;97(6):e0005923. doi: 10.1128/jvi.00059-23. Epub 2023 May 31. J Virol. 2023. PMID: 37255431 Free PMC article.
References
-
- Albright A. V., Shieh J. T., O’Connor M. J., Gonzalez-Scarano F. (2000). Characterization of cultured microglia that can be infected by HIV-1. J. Neurovirol. 6 S53–S60. - PubMed
-
- Almeida O. P., Lautenschlager N. T. (2005). Dementia associated with infectious diseases. Int. Psychogeriatr. 17 S65–S77. - PubMed
-
- Alvarez-Lafuente R., Garcia-Montojo M., De Las Heras V., Dominguez-Mozo M. I., Bartolome M., Benito-Martin M. S., et al. (2008). Herpesviruses and human endogenous retroviral sequences in the cerebrospinal fluid of multiple sclerosis patients. Mult. Scler 14 595–601. 10.1177/1352458507086425 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

