Chronic Myeloid Leukemia: Modern therapies, current challenges and future directions
- PMID: 33773846
- PMCID: PMC8563059
- DOI: 10.1016/j.blre.2021.100825
Chronic Myeloid Leukemia: Modern therapies, current challenges and future directions
Abstract
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm caused by a reciprocal translocation [t(9;22)(q34;q11.2)] that leads to the fusion of ABL1 gene sequences (9q34) downstream of BCR gene sequences (22q11) and is cytogenetically visible as Philadelphia chromosome (Ph). The resulting BCR/ABL1 chimeric protein is a constitutively active tyrosine kinase that activates multiple signaling pathways, which collectively lead to malignant transformation. During the early (chronic) phase of CML (CP-CML), the myeloid cell compartment is expanded, but differentiation is maintained. Without effective therapy, CP-CML invariably progresses to blast phase (BP-CML), an acute leukemia of myeloid or lymphoid phenotype. The development of BCR-AB1 tyrosine kinase inhibitors (TKIs) revolutionized the treatment of CML and ignited the start of a new era in oncology. With three generations of BCR/ABL1 TKIs approved today, the majority of CML patients enjoy long term remissions and near normal life expectancy. However, only a minority of patients maintain remission after TKI discontinuation, a status termed treatment free remission (TFR). Unfortunately, 5-10% of patients fail TKIs due to resistance and are at risk of progression to BP-CML, which is curable only with hematopoietic stem cell transplantation. Overcoming TKI resistance, improving the prognosis of BP-CML and improving the rates of TFR are areas of active research in CML.
Keywords: BCR/ABL1; Chronic myeloid leukemia; Philadelphia chromosome; Treatment free remission; Tyrosine kinase inhibitor.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement
No conflicts of interest, financial or other, exist for Afaf Osman. Michael Deininger’s potential conflicts of interest includes serving on the advisory board of Blueprint, Takeda, Novartis, Incyte, Sangamo and Pfizer, paid consultation at Blueprint, Fusion Pharma, Medscape, Novartis, Sangamo, DisperSol and the NCCN, research funding from Blueprint, Takeda, Novartis, Incyte, SPARC, Leukemia and Lymphoma Society and Pfizer and study management committees at Blueprint and Takeda. He is a case author at Medscape.
Figures
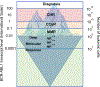

Similar articles
-
Chronic myeloid leukemia: 2025 update on diagnosis, therapy, and monitoring.Am J Hematol. 2024 Nov;99(11):2191-2212. doi: 10.1002/ajh.27443. Epub 2024 Aug 2. Am J Hematol. 2024. PMID: 39093014 Review.
-
Chronic myeloid leukemia: 2022 update on diagnosis, therapy, and monitoring.Am J Hematol. 2022 Sep;97(9):1236-1256. doi: 10.1002/ajh.26642. Epub 2022 Jul 6. Am J Hematol. 2022. PMID: 35751859
-
Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring.Am J Hematol. 2020 Jun;95(6):691-709. doi: 10.1002/ajh.25792. Epub 2020 Apr 10. Am J Hematol. 2020. PMID: 32239758
-
Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring.Am J Hematol. 2018 Mar;93(3):442-459. doi: 10.1002/ajh.25011. Am J Hematol. 2018. PMID: 29411417 Review.
-
Chronic myeloid leukemia: 2016 update on diagnosis, therapy, and monitoring.Am J Hematol. 2016 Feb;91(2):252-65. doi: 10.1002/ajh.24275. Am J Hematol. 2016. PMID: 26799612
Cited by
-
Trends and prescribing patterns of oral anti-neoplastic drugs: a retrospective longitudinal study.Front Public Health. 2023 Nov 23;11:1294126. doi: 10.3389/fpubh.2023.1294126. eCollection 2023. Front Public Health. 2023. PMID: 38074729 Free PMC article.
-
Molecular subtypes predict therapeutic responses and identifying and validating diagnostic signatures based on machine learning in chronic myeloid leukemia.Cancer Cell Int. 2023 Apr 6;23(1):61. doi: 10.1186/s12935-023-02905-x. Cancer Cell Int. 2023. PMID: 37024911 Free PMC article.
-
DNA binding, and apoptosis-inducing activities of a β-ionone-derived ester in human myeloid leukemia cells: multispectral and molecular dynamic simulation analyses.Sci Rep. 2024 Nov 14;14(1):27985. doi: 10.1038/s41598-024-78690-y. Sci Rep. 2024. PMID: 39543249 Free PMC article.
-
Beyond IC50-A computational dynamic model of drug resistance in enzyme inhibition treatment.PLoS Comput Biol. 2024 Nov 7;20(11):e1012570. doi: 10.1371/journal.pcbi.1012570. eCollection 2024 Nov. PLoS Comput Biol. 2024. PMID: 39509464 Free PMC article.
-
SKF-96365 Expels Tyrosine Kinase Inhibitor-Treated CML Stem and Progenitor Cells from the HS27A Stromal Cell Niche in a RhoA-Dependent Mechanism.Cancers (Basel). 2024 Aug 8;16(16):2791. doi: 10.3390/cancers16162791. Cancers (Basel). 2024. PMID: 39199564 Free PMC article.
References
-
- Gambacorti-Passerini C, Antolini L, Mahon FX, Guilhot F, Deininger M, Fava C, et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst. 2011;103(7):553–61. - PubMed
-
- Ichimaru M, Tomonaga M, Amenomori T, Matsuo T. Atomic bomb and leukemia. J Radiat Res. 1991;32 Suppl:162–7. - PubMed
-
- Little MP, Weiss HA, Boice JD, Jr., Darby SC, Day NE, Muirhead CR. Risks of leukemia in Japanese atomic bomb survivors, in women treated for cervical cancer, and in patients treated for ankylosing spondylitis. Radiat Res. 1999;152(3):280–92. - PubMed
-
- Van Kaick G, Wesch H, Luhrs H, Liebermann D, Kaul A. Neoplastic diseases induced by chronic alpha-irradiation-- epidemiological, biophysical and clinical results of the German Thorotrast Study. JRadiatResTokyo. 1991;32 Suppl 2:20–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

