Emerging roles of circular RNAs in systemic lupus erythematosus
- PMID: 33767917
- PMCID: PMC7973136
- DOI: 10.1016/j.omtn.2021.02.028
Emerging roles of circular RNAs in systemic lupus erythematosus
Abstract
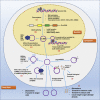
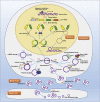
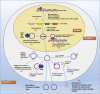
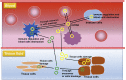
Circular RNAs (circRNAs) are a class of non-coding RNAs with covalently closed single-stranded structures lacking 5'-3' polarity and a polyadenine tail. Over recent years, a growing body of studies have been conducted to explore the roles of circRNAs in human diseases. Systemic lupus erythematosus (SLE) is a severe autoimmune disorder characterized by the presence of autoantibodies and excessive inflammation, which impact multiple organs. Recent advances have begun to shed light on the roles of circRNAs in SLE, providing fresh insights into the pathogenesis of SLE and the latent capacity for translation into clinical applications. Here, we briefly introduce these "star molecules" and summarize their roles in SLE. In addition, we outline the limitations of the current studies and raise prospects for future research.
Keywords: circular RNA; expression; function; mechanism; systemic lupus erythematosus.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Circular RNAs and systemic lupus erythematosus.Exp Cell Res. 2016 Aug 15;346(2):248-54. doi: 10.1016/j.yexcr.2016.07.021. Epub 2016 Jul 21. Exp Cell Res. 2016. PMID: 27450756 Review.
-
Current Understanding of Circular RNAs in Systemic Lupus Erythematosus.Front Immunol. 2021 Feb 25;12:628872. doi: 10.3389/fimmu.2021.628872. eCollection 2021. Front Immunol. 2021. PMID: 33717154 Free PMC article. Review.
-
Circular RNA in autoimmune diseases: special emphasis on regulation mechanism in RA and SLE.J Pharm Pharmacol. 2023 Mar 12;75(3):370-384. doi: 10.1093/jpp/rgac096. J Pharm Pharmacol. 2023. PMID: 36583516
-
Microarray expression profile of circular RNAs and mRNAs in children with systemic lupus erythematosus.Clin Rheumatol. 2019 May;38(5):1339-1350. doi: 10.1007/s10067-018-4392-8. Epub 2019 Jan 9. Clin Rheumatol. 2019. PMID: 30628013
-
RNA-seq of circular RNAs identified circPTPN22 as a potential new activity indicator in systemic lupus erythematosus.Lupus. 2019 Apr;28(4):520-528. doi: 10.1177/0961203319830493. Epub 2019 Mar 14. Lupus. 2019. PMID: 30871426
Cited by
-
Identification of circular RNAs of Cannabis sativa L. potentially involved in the biosynthesis of cannabinoids.Planta. 2023 Mar 2;257(4):72. doi: 10.1007/s00425-023-04104-4. Planta. 2023. PMID: 36862222
-
The role of hsa_circ_0008945 in juvenile-onset systemic lupus erythematosus.BMC Med Genomics. 2023 May 9;16(1):97. doi: 10.1186/s12920-023-01524-9. BMC Med Genomics. 2023. PMID: 37161408 Free PMC article.
-
A RBM47 and IGF2BP1 mediated circular FNDC3B-FNDC3B mRNA imbalance is involved in the malignant processes of osteosarcoma.Cancer Cell Int. 2023 Dec 21;23(1):334. doi: 10.1186/s12935-023-03175-3. Cancer Cell Int. 2023. PMID: 38129874 Free PMC article.
-
Research Techniques Made Simple: Studying Circular RNA in Skin Diseases.J Invest Dermatol. 2021 Oct;141(10):2313-2319.e1. doi: 10.1016/j.jid.2021.07.156. J Invest Dermatol. 2021. PMID: 34560913 Free PMC article. Review.
-
circRNA: Regulatory factors and potential therapeutic targets in inflammatory dermatoses.J Cell Mol Med. 2022 Aug;26(16):4389-4400. doi: 10.1111/jcmm.17473. Epub 2022 Jun 29. J Cell Mol Med. 2022. PMID: 35770323 Free PMC article. Review.
References
-
- Dörner T., Furie R. Novel paradigms in systemic lupus erythematosus. Lancet. 2019;393:2344–2358. - PubMed
-
- Durcan L., O’Dwyer T., Petri M. Management strategies and future directions for systemic lupus erythematosus in adults. Lancet. 2019;393:2332–2343. - PubMed
-
- Tsokos G.C., Lo M.S., Costa Reis P., Sullivan K.E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2016;12:716–730. - PubMed
-
- Mohan C., Putterman C. Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis. Nat. Rev. Nephrol. 2015;11:329–341. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

