Macrophages and microglia: the cerberus of glioblastoma
- PMID: 33766119
- PMCID: PMC7992800
- DOI: 10.1186/s40478-021-01156-z
Macrophages and microglia: the cerberus of glioblastoma
Abstract
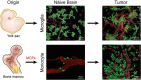
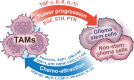
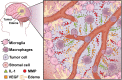
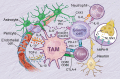
Glioblastoma (GBM) is the most aggressive and deadliest of the primary brain tumors, characterized by malignant growth, invasion into the brain parenchyma, and resistance to therapy. GBM is a heterogeneous disease characterized by high degrees of both inter- and intra-tumor heterogeneity. Another layer of complexity arises from the unique brain microenvironment in which GBM develops and grows. The GBM microenvironment consists of neoplastic and non-neoplastic cells. The most abundant non-neoplastic cells are those of the innate immune system, called tumor-associated macrophages (TAMs). TAMs constitute up to 40% of the tumor mass and consist of both brain-resident microglia and bone marrow-derived myeloid cells from the periphery. Although genetically stable, TAMs can change their expression profiles based upon the signals that they receive from tumor cells; therefore, heterogeneity in GBM creates heterogeneity in TAMs. By interacting with tumor cells and with the other non-neoplastic cells in the tumor microenvironment, TAMs promote tumor progression. Here, we review the origin, heterogeneity, and functional roles of TAMs. In addition, we discuss the prospects of therapeutically targeting TAMs alone or in combination with standard or newly-emerging GBM targeting therapies.
Keywords: Glioblastoma; Heterogeneity; Macrophages; Microenvironment; Microglia.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Immune Microenvironment in Glioblastoma Subtypes.Front Immunol. 2018 May 8;9:1004. doi: 10.3389/fimmu.2018.01004. eCollection 2018. Front Immunol. 2018. PMID: 29867979 Free PMC article. Review.
-
Neuroinflammation in Glioblastoma: The Role of the Microenvironment in Tumour Progression.Curr Cancer Drug Targets. 2024;24(6):579-594. doi: 10.2174/0115680096265849231031101449. Curr Cancer Drug Targets. 2024. PMID: 38310461 Review.
-
Enhancing Glioblastoma Immunotherapy with Integrated Chimeric Antigen Receptor T Cells through the Re-Education of Tumor-Associated Microglia and Macrophages.ACS Nano. 2024 Apr 30;18(17):11165-11182. doi: 10.1021/acsnano.4c00050. Epub 2024 Apr 16. ACS Nano. 2024. PMID: 38626338
-
Distinct regional ontogeny and activation of tumor associated macrophages in human glioblastoma.Sci Rep. 2020 Nov 11;10(1):19542. doi: 10.1038/s41598-020-76657-3. Sci Rep. 2020. PMID: 33177572 Free PMC article.
-
GBM Immunotherapy: Macrophage Impacts.Immunol Invest. 2024 Jul;53(5):730-751. doi: 10.1080/08820139.2024.2337022. Epub 2024 Apr 18. Immunol Invest. 2024. PMID: 38634572 Review.
Cited by
-
The road we travel.Neuro Oncol. 2023 Jan 5;25(1):135-136. doi: 10.1093/neuonc/noac235. Neuro Oncol. 2023. PMID: 36219132 Free PMC article. No abstract available.
-
Neutrophil heterogeneity and aging: implications for COVID-19 and wound healing.Front Immunol. 2023 Nov 28;14:1201651. doi: 10.3389/fimmu.2023.1201651. eCollection 2023. Front Immunol. 2023. PMID: 38090596 Free PMC article. Review.
-
Glioblastoma: Pitfalls and Opportunities of Immunotherapeutic Combinations.Onco Targets Ther. 2022 Apr 28;15:437-468. doi: 10.2147/OTT.S215997. eCollection 2022. Onco Targets Ther. 2022. PMID: 35509452 Free PMC article. Review.
-
Comprehensive Analysis Identified Glycosyltransferase Signature to Predict Glioma Prognosis and TAM Phenotype.Biomed Res Int. 2023 Jan 11;2023:6082635. doi: 10.1155/2023/6082635. eCollection 2023. Biomed Res Int. 2023. PMID: 36685667 Free PMC article.
-
TGFBI secreted by tumor-associated macrophages promotes glioblastoma stem cell-driven tumor growth via integrin αvβ5-Src-Stat3 signaling.Theranostics. 2022 May 16;12(9):4221-4236. doi: 10.7150/thno.69605. eCollection 2022. Theranostics. 2022. PMID: 35673564 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

