Biomarkers of lesion severity in a rodent model of nonarteritic anterior ischemic optic neuropathy (rNAION)
- PMID: 33764998
- PMCID: PMC7993789
- DOI: 10.1371/journal.pone.0243186
Biomarkers of lesion severity in a rodent model of nonarteritic anterior ischemic optic neuropathy (rNAION)
Abstract
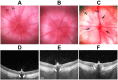
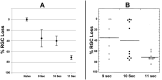
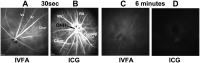
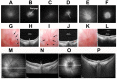
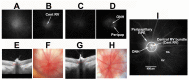
The rodent model of nonarteritic anterior ischemic optic neuropathy (rNAION) is similar in many of its pathophysiological responses to clinical NAION. Like human NAION, there is significant variability in the severity of the lesion produced, and little is known of the parameters associated with rNAION induction severity or if pre- or early post-induction biomarkers can be identified that enable prediction of lesion severity and ultimate loss of retinal ganglion cells (RGCs). Adult male Sprague-Dawley outbred rats were evaluated for various parameters including physiological characteristics (heart rate, respiratory rate, temperature, hematocrit [Hct]), optic nerve head (ONH) appearance, pre- and post-induction mean diameter, and intravenous fluorescein and indocyanine green angiographic patterns of vascular leakage at 5 hours post-induction, performed using a spectral domain-optical coherence tomography (SD-OCT) instrument. Early changes were correlated with ultimate RGC loss by Brn3a (+) immunohistology. RGC loss also was correlated with the relative level of laser exposure. The severity of ONH edema 2d, but not 5hr, post induction was most closely associated with the degree of RGC loss, revealing a threshold effect, and consistent with a compartment syndrome where a minimum level of capillary compression within a tight space is responsible for damage. RGC loss increased dramatically as the degree of laser exposure increased. Neither physiological parameters nor the degree of capillary leakage 5hr post induction were informative as to the ultimate degree of RGC loss. Similar to human NAION, the rNAION model exhibits marked variability in lesion severity. Unlike clinical NAION, pre-induction ONH diameter likely does not contribute to ultimate lesion severity; however, cross-sectional ONH edema can be used as a biomarker 2d post-induction to determine randomization of subjects prior to inclusion in specific neuroprotection or neuroregeneration studies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
[Nonarteritic ischemic optic neuropathy animal model and its treatment applications].Nippon Ganka Gakkai Zasshi. 2014 Apr;118(4):331-61. Nippon Ganka Gakkai Zasshi. 2014. PMID: 24864434 Review. Japanese.
-
Oligodendrocyte death, neuroinflammation, and the effects of minocycline in a rodent model of nonarteritic anterior ischemic optic neuropathy (rNAION).Mol Vis. 2017 Dec 17;23:963-976. eCollection 2017. Mol Vis. 2017. PMID: 29386871 Free PMC article.
-
[Temporal and spatial characteristics of RGC death and axon degeneration in the rat model of nonarteritic anterior ischemic optic neuropathy].Zhonghua Yan Ke Za Zhi. 2016 Dec 11;52(12):918-923. doi: 10.3760/cma.j.issn.0412-4081.2016.12.009. Zhonghua Yan Ke Za Zhi. 2016. PMID: 27998456 Chinese.
-
Longitudinal Measurement of Hemodynamic Changes within the Posterior Optic Nerve Head in Rodent Nonarteritic Anterior Ischemic Optic Neuropathy.Chin Med Sci J. 2018 Dec 30;33(4):252-259. doi: 10.24920/003477. Chin Med Sci J. 2018. PMID: 30646989
-
Nonarteritic anterior ischemic optic neuropathy (NAION) and its experimental models.Prog Retin Eye Res. 2011 May;30(3):167-87. doi: 10.1016/j.preteyeres.2011.02.003. Epub 2011 Mar 2. Prog Retin Eye Res. 2011. PMID: 21376134 Free PMC article. Review.
Cited by
-
Synergistic Protection of Retinal Ganglion Cells (RGCs) by SARM1 Inactivation with CNTF in a Rodent Model of Nonarteritic Anterior Ischemic Optic Neuropathy.Cells. 2024 Jan 23;13(3):202. doi: 10.3390/cells13030202. Cells. 2024. PMID: 38334594 Free PMC article.
-
Non-Arteritic Anterior Ischemic Optic Neuropathy: Challenges for the Future.Front Ophthalmol (Lausanne). 2022 Mar 15;2:848710. doi: 10.3389/fopht.2022.848710. eCollection 2022. Front Ophthalmol (Lausanne). 2022. PMID: 38983540 Free PMC article. No abstract available.
-
Neuroprotection and Neuroregeneration Strategies Using the rNAION Model: Theory, Histology, Problems, Results and Analytical Approaches.Int J Mol Sci. 2022 Dec 9;23(24):15604. doi: 10.3390/ijms232415604. Int J Mol Sci. 2022. PMID: 36555246 Free PMC article.
-
Anti-NOGO Antibody Neuroprotection in a Rat Model of NAION.Transl Vis Sci Technol. 2021 Dec 1;10(14):12. doi: 10.1167/tvst.10.14.12. Transl Vis Sci Technol. 2021. PMID: 34904998 Free PMC article.
-
Repeat Brn3a immunolabeling rescues faded staining and improves detection of retinal ganglion cells.Exp Eye Res. 2023 Jan;226:109310. doi: 10.1016/j.exer.2022.109310. Epub 2022 Nov 15. Exp Eye Res. 2023. PMID: 36400286 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

