Tumor DNA methylation profiles correlate with response to anti-PD-1 immune checkpoint inhibitor monotherapy in sarcoma patients
- PMID: 33762319
- PMCID: PMC7993298
- DOI: 10.1136/jitc-2020-001458
Tumor DNA methylation profiles correlate with response to anti-PD-1 immune checkpoint inhibitor monotherapy in sarcoma patients
Abstract
Background: Some sarcomas respond to immune checkpoint inhibition, but predictive biomarkers are unknown. We analyzed tumor DNA methylation profiles in relation to immunological parameters and response to anti-programmed cell death 1 (anti-PD-1) immune checkpoint inhibitor (ICI) therapy in patients with sarcoma.
Patients and methods: We retrospectively identified adult patients who had received anti-PD-1 ICI therapy for recurrent sarcoma in two independent centers. We performed (1) blinded radiological response evaluation according to immune response evaluation criteria in solid tumors (iRECIST) ; (2) tumor DNA methylation profiling of >850,000 probes using Infinium MethylationEPIC microarrays; (3) analysis of tumor-infiltrating immune cell subsets (CD3, CD8, CD45RO, FOXP3) and intratumoral expression of immune checkpoint molecules (PD-L1, PD-1, LAG-3) using immunohistochemistry; and (4) evaluation of blood-based systemic inflammation scores (neutrophil-to-lymphocyte ratio, leucocyte-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio). Response to anti-PD-1 ICI therapy was bioinformatically and statistically correlated with DNA methylation profiles and immunological data.
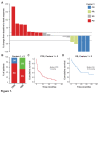
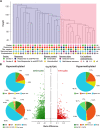
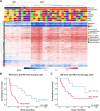
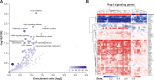
Results: 35 patients (median age of 50 (23-81) years; 18 females, 17 males; 27 soft tissue sarcomas; 8 osteosarcomas) were included in this study. The objective response rate to anti-PD-1 ICI therapy was 22.9% with complete responses in 3 out of 35 and partial responses in 5 out of 35 patients. Adjustment of DNA methylation data for tumor-infiltrating immune cells resulted in identification of methylation differences between responders and non-responders to anti-PD-1 ICI. 2453 differentially methylated CpG sites (DMPs; 2043 with decreased and 410 with increased methylation) were identified. Clustering of sarcoma samples based on these DMPs revealed two main clusters: methylation cluster 1 (MC1) consisted of 73% responders and methylation cluster 2 (MC2) contained only non-responders to anti-PD-1 ICI. Median progression-free survival from anti-PD-1 therapy start of MC1 and MC2 patients was 16.5 and 1.9 months, respectively (p=0.001). Median overall survival of these patients was 34.4 and 8.0 months, respectively (p=0.029). The most prominent DNA methylation differences were found in pathways implicated in Rap1 signaling, focal adhesion, adherens junction Phosphoinositide 3-kinase (PI3K)-Akt signaling and extracellular matrix (ECM)-receptor interaction.
Conclusions: Our data demonstrate that tumor DNA methylation profiles may serve as a predictive marker for response to anti-PD-1 ICI therapy in sarcoma.
Keywords: biomarkers; biostatistics; immunotherapy; sarcoma; tumor.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: AS has received travel support from PharmaMar. AB has research support from Daiichi Sankyo and Roche; honoraria for lectures, consultation or advisory board participation from Roche Bristol-Meyers Squibb, Merck, Daiichi Sankyo; as well as travel support from Roche, Amgen, Daiichi Sankyo and AbbVie. MP has received honoraria for lectures, consultation or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, Tocagen. The following for-profit companies have supported clinical trials and contracted research conducted by MP with payments made to his institution: Böhringer-Ingelheim, Bristol-Myers Squibb, Roche, Daiichi Sankyo, Merck Sharp & Dome, Novocure, GlaxoSmithKline, AbbVie. TB reports personal fees from Roche (lecture fee), personal fees from Amgen (lecture fee, advisory board), personal fees from Bayer (lecture fee, advisory board), personal fees from Novartis (lecture fee, advisory board), personal fees from PharmaMar (lecture fee, advisory board), personal fees from Eisai (lecture fee, advisory board), personal fees from Eli Lilly (lecture fee, advisory board), outside the submitted work. RH is supported by Clinician Scientist Program of the University Medicine Essen Clinician Scientist Academy (UMEA) sponsored by faculty of medicine and Deutsche Forschungsgemeinschaft (DFG). RH has received travel grants from Lilly, Novartis and PharmaMar, as well as fees from Lilly outside of the submitted work. SB reports personal fees from Deciphera, grants from Incyte, grants and personal fees from Blueprint Medicines, personal fees from Lilly, grants and personal fees from Novartis, personal fees from Daichii-Sankyo, personal fees from Plexxikon, personal fees from Exelixis, personal fees from Bayer, other from Pfizer, during the conduct of the study; personal fees from Pharmamar, personal fees from Lilly, personal fees from Roche, personal fees from GSK, outside the submitted work. All other authors report no conflicts of interest concerning this specific publication.
Figures
Similar articles
-
DNA methylation profiles differ in responders versus non-responders to anti-PD-1 immune checkpoint inhibitors in patients with advanced and metastatic head and neck squamous cell carcinoma.J Immunother Cancer. 2022 Mar;10(3):e003420. doi: 10.1136/jitc-2021-003420. J Immunother Cancer. 2022. PMID: 35338086 Free PMC article.
-
Correlative Analyses of the SARC028 Trial Reveal an Association Between Sarcoma-Associated Immune Infiltrate and Response to Pembrolizumab.Clin Cancer Res. 2020 Mar 15;26(6):1258-1266. doi: 10.1158/1078-0432.CCR-19-1824. Epub 2020 Jan 3. Clin Cancer Res. 2020. PMID: 31900276 Free PMC article. Clinical Trial.
-
Pathways of immune exclusion in metastatic osteosarcoma are associated with inferior patient outcomes.J Immunother Cancer. 2021 May;9(5):e001772. doi: 10.1136/jitc-2020-001772. J Immunother Cancer. 2021. PMID: 34021032 Free PMC article.
-
Improving Immunotherapy Efficacy in Soft-Tissue Sarcomas: A Biomarker Driven and Histotype Tailored Review.Front Immunol. 2021 Dec 3;12:775761. doi: 10.3389/fimmu.2021.775761. eCollection 2021. Front Immunol. 2021. PMID: 34925348 Free PMC article. Review.
-
Therapeutic Targets for Bone and Soft-Tissue Sarcomas.Int J Mol Sci. 2019 Jan 4;20(1):170. doi: 10.3390/ijms20010170. Int J Mol Sci. 2019. PMID: 30621224 Free PMC article. Review.
Cited by
-
Impact of anthracycline-based chemotherapy on RB1 gene methylation in peripheral blood leukocytes and biomarkers of oxidative stress and inflammation in sarcoma patients.Clin Transl Oncol. 2024 Jun;26(6):1508-1518. doi: 10.1007/s12094-023-03375-3. Epub 2024 Feb 3. Clin Transl Oncol. 2024. PMID: 38310203
-
Advances in Osteosarcoma.Curr Osteoporos Rep. 2023 Aug;21(4):330-343. doi: 10.1007/s11914-023-00803-9. Epub 2023 Jun 17. Curr Osteoporos Rep. 2023. PMID: 37329384 Free PMC article. Review.
-
DNA methylation profiles differ in responders versus non-responders to anti-PD-1 immune checkpoint inhibitors in patients with advanced and metastatic head and neck squamous cell carcinoma.J Immunother Cancer. 2022 Mar;10(3):e003420. doi: 10.1136/jitc-2021-003420. J Immunother Cancer. 2022. PMID: 35338086 Free PMC article.
-
Exploring the landscape of immunotherapy approaches in sarcomas.Front Oncol. 2023 Jan 9;12:1069963. doi: 10.3389/fonc.2022.1069963. eCollection 2022. Front Oncol. 2023. PMID: 36686827 Free PMC article. Review.
-
Biomarkers and experimental models for cancer immunology investigation.MedComm (2020). 2023 Dec 2;4(6):e437. doi: 10.1002/mco2.437. eCollection 2023 Dec. MedComm (2020). 2023. PMID: 38045830 Free PMC article. Review.
References
-
- Fletcher CDM, Hogendoorn P, Mertens F. WHO classification of tumors of soft tissue and bone. Lyon IARC Press, 2013: 321–4.
-
- Demetri GD, von Mehren M, Jones RL, et al. . Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol 2016;34:786–93. 10.1200/JCO.2015.62.4734 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
