Efficacy and safety of single-inhaler extrafine triple therapy versus inhaled corticosteroid plus long-acting beta2 agonist in eastern Asian patients with COPD: the TRIVERSYTI randomised controlled trial
- PMID: 33757520
- PMCID: PMC7989027
- DOI: 10.1186/s12931-021-01683-2
Efficacy and safety of single-inhaler extrafine triple therapy versus inhaled corticosteroid plus long-acting beta2 agonist in eastern Asian patients with COPD: the TRIVERSYTI randomised controlled trial
Abstract
Background: A single-inhaler extrafine triple combination of beclometasone dipropionate (BDP), formoterol fumarate (FF) and glycopyrronium (G) has been developed for maintenance therapy of chronic obstructive pulmonary disease (COPD). This study evaluated the efficacy and safety of BDP/FF/G in patients in three eastern Asian areas: China, Republic of Korea and Taiwan.
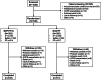
Methods: TRIVERSYTI was a double-blind, randomised, active-controlled, parallel-group study in patients with COPD, post-bronchodilator forced expiratory volume in 1 s (FEV1) < 50% predicted, ≥ 1 exacerbation in the previous 12 months, and receiving inhaled maintenance medication. Patients received either extrafine BDP/FF/G 100/6/10 µg via pressurised metered-dose inhaler, or non-extrafine budesonide/formoterol (BUD/FF) 160/4.5 µg via dry-powder inhaler, both administered as two puffs twice-daily for 24 weeks. The co-primary objectives (analysed in the overall population) were to demonstrate superiority of BDP/FF/G over BUD/FF for change from baseline in pre-dose morning and 2-h post-dose FEV1 at Week 24 (these were analysed as key secondary objectives in the China subgroup). The rate of moderate/severe COPD exacerbations was a secondary endpoint.
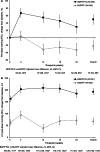
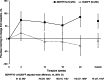
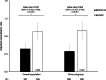
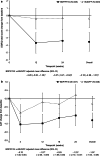
Results: Of 708 patients randomised, 88.8% completed. BDP/FF/G was superior to BUD/FF for pre-dose and 2-h post-dose FEV1 at Week 24 [adjusted mean differences 62 (95% CI 38, 85) mL and 113 (87, 140) mL; both p < 0.001]. The annualised moderate/severe exacerbation rate was 43% lower with BDP/FF/G [rate ratio 0.57 (95% CI 0.42, 0.77); p < 0.001]. Adverse events were reported by 61.1% and 67.0% patients with BDP/FF/G and BUD/FF. Results were similar in the China subgroup.
Conclusions: In patients with COPD, FEV1 < 50% and an exacerbation history despite maintenance therapy, treatment with extrafine BDP/FF/G improved bronchodilation, and was more effective at preventing moderate/severe COPD exacerbations than BUD/FF. Trial registration CFDA CTR20160507 (registered 7 Nov 2016, http://www.chinadrugtrials.org.cn/index.html ).
Keywords: Airway obstruction; Chronic bronchitis; Chronic obstructive pulmonary disease; Extrafine; Triple inhalation therapy.
Conflict of interest statement
JZ has no relevant conflicts to disclose. LZ has no relevant conflicts to disclose. HL has no relevant conflicts to disclose. K-HL has no relevant conflicts to disclose. DS reports personal fees from Chiesi during the conduct of the study. Outside the submitted work, he reports personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Genentech, GlaxoSmithKline, Glenmark, Menarini, Mundipharma, Novartis, Peptinnovate, Pfizer, Pulmatrix, Theravance, and Verona. AP reports grants, personal fees, non-financial support and payment for advisory board membership, consultancy, payment for lectures, grants for research, and travel expenses reimbursement from Chiesi, AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Mundipharma and TEVA, and personal fees and non-financial support from Menarini, Novartis, Zambon and Sanofi, all outside the submitted work. SB, FG, AG and GG are employees of Chiesi, the sponsor of the study.
Figures
Similar articles
-
Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial.Lancet. 2018 Mar 17;391(10125):1076-1084. doi: 10.1016/S0140-6736(18)30206-X. Epub 2018 Feb 9. Lancet. 2018. PMID: 29429593 Clinical Trial.
-
The TRIFLOW study: a randomised, cross-over study evaluating the effects of extrafine beclometasone/formoterol/glycopyrronium on gas trapping in COPD.Respir Res. 2020 Dec 9;21(1):323. doi: 10.1186/s12931-020-01589-5. Respir Res. 2020. PMID: 33298062 Free PMC article. Clinical Trial.
-
The bronchodilator effects of extrafine glycopyrronium added to combination treatment with beclometasone dipropionate plus formoterol in COPD: A randomised crossover study (the TRIDENT study).Respir Med. 2016 May;114:84-90. doi: 10.1016/j.rmed.2016.03.018. Epub 2016 Mar 26. Respir Med. 2016. PMID: 27109816 Clinical Trial.
-
Triple therapy in COPD: new evidence with the extrafine fixed combination of beclomethasone dipropionate, formoterol fumarate, and glycopyrronium bromide.Int J Chron Obstruct Pulmon Dis. 2017 Oct 6;12:2917-2928. doi: 10.2147/COPD.S146822. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 29062229 Free PMC article. Review.
-
Spotlight on glycopyrronium/formoterol fumarate inhalation aerosol in the management of COPD: design, development, and place in therapy.Int J Chron Obstruct Pulmon Dis. 2017 Aug 3;12:2307-2312. doi: 10.2147/COPD.S89482. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 28814858 Free PMC article. Review.
Cited by
-
Smoking cessation and vaccination.Eur Respir Rev. 2023 Mar 22;32(167):220187. doi: 10.1183/16000617.0187-2022. Print 2023 Mar 31. Eur Respir Rev. 2023. PMID: 36948500 Free PMC article.
-
Intraclass comparison of inhaled corticosteroids for the risk of pneumonia in chronic obstructive pulmonary airway disorder: a network meta-analysis and meta-regression.Int J Clin Pharm. 2024 Aug;46(4):831-842. doi: 10.1007/s11096-024-01736-8. Epub 2024 Apr 25. Int J Clin Pharm. 2024. PMID: 38664319
-
Extrafine single inhaler triple therapy effectiveness in COPD patients previously treated with multiple-inhaler triple therapy: the TRIWIN study.Ther Adv Respir Dis. 2024 Jan-Dec;18:17534666241263439. doi: 10.1177/17534666241263439. Ther Adv Respir Dis. 2024. PMID: 39049587 Free PMC article.
-
Different inhaled corticosteroid doses in triple therapy for chronic obstructive pulmonary disease: systematic review and Bayesian network meta-analysis.Sci Rep. 2022 Sep 20;12(1):15698. doi: 10.1038/s41598-022-18353-y. Sci Rep. 2022. PMID: 36127353 Free PMC article.
-
The clinical characteristics and outcomes of different inhaled therapies in chronic obstructive pulmonary disease patients with frequent cough.Ann Med. 2023;55(2):2304107. doi: 10.1080/07853890.2024.2304107. Epub 2024 Jan 17. Ann Med. 2023. PMID: 38233371 Free PMC article.
References
-
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease [Internet]. 2021 [cited 2020 Nov 25]. https://goldcopd.org/2021-gold-reports/.
-
- Braido F, Scichilone N, Lavorini F, Usmani OS, Dubuske L, Boulet LP, et al. Manifesto on small airway involvement and management in asthma and chronic obstructive pulmonary disease: an Interasma (Global Asthma Association - GAA) and World Allergy Organization (WAO) document. World Allergy Organ J. 2016;9:1–6. doi: 10.1186/s40413-016-0123-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

