MicroRNA-146a switches microglial phenotypes to resist the pathological processes and cognitive degradation of Alzheimer's disease
- PMID: 33754051
- PMCID: PMC7977456
- DOI: 10.7150/thno.53418
MicroRNA-146a switches microglial phenotypes to resist the pathological processes and cognitive degradation of Alzheimer's disease
Abstract
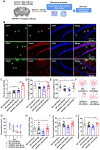
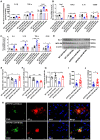
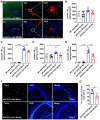
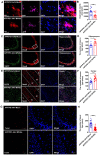
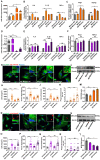
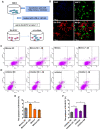
Alzheimer's disease (AD) is the most prevalent neurodegenerative disease and currently has no effective treatment. Mainstream research on the mechanisms and therapeutic targets of AD is focused on the two most important hallmarks, Aβ and Tau, but the results from clinical studies are not encouraging. Abnormal microglial polarization is a clear typical pathological feature in the progression of AD. Microglia can be neuroprotective by degrading and removing Aβ and Tau. However, under AD conditions, microglia transform into a pro-inflammatory phenotype that decreases the phagocytic activity of microglia, damages neurons and promotes the pathology of AD. We previously reported that a miR-146a polymorphism is associated with sporadic AD risk, and the nasal administration of miR-146a mimics reduced cognitive impairment and the main pathological features of AD. However, it is not clear by what mechanism miR-146a resists the pathological process of AD. In this study, we discovered that microglia-specific miR-146a overexpression reduced cognitive deficits in learning and memory, attenuated neuroinflammation, reduced Aβ levels, ameliorated plaque-associated neuritic pathology, and prevented neuronal loss in APP/PS1 transgenic mice. In addition, we found that miR-146a switched the microglial phenotype, reduced pro-inflammatory cytokines and enhanced phagocytic function to protect neurons in vitro and in vivo. Moreover, transcriptional analysis confirmed that miR-146a opposed the pathological process of AD mainly through neuroinflammation-related pathways. In summary, our results provide sufficient evidence for the mechanism by which miR-146a opposes AD and strengthen the conclusion that miR-146a is a promising target for AD and other microglia-related diseases.
Keywords: Alzheimer's disease; microRNA-146a; microglial polarization, neuroinfammation, phagocytic activity..
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
p110δ PI3-Kinase Inhibition Perturbs APP and TNFα Trafficking, Reduces Plaque Burden, Dampens Neuroinflammation, and Prevents Cognitive Decline in an Alzheimer's Disease Mouse Model.J Neurosci. 2019 Oct 2;39(40):7976-7991. doi: 10.1523/JNEUROSCI.0674-19.2019. Epub 2019 Jul 30. J Neurosci. 2019. PMID: 31363064 Free PMC article.
-
Fibrillar Aβ triggers microglial proteome alterations and dysfunction in Alzheimer mouse models.Elife. 2020 Jun 8;9:e54083. doi: 10.7554/eLife.54083. Elife. 2020. PMID: 32510331 Free PMC article.
-
Knockdown of microglial iron import gene, Slc11a2, worsens cognitive function and alters microglial transcriptional landscape in a sex-specific manner in the APP/PS1 model of Alzheimer's disease.J Neuroinflammation. 2024 Sep 27;21(1):238. doi: 10.1186/s12974-024-03238-w. J Neuroinflammation. 2024. PMID: 39334471 Free PMC article.
-
Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
-
MicroRNA-455-5p/CPEB1 pathway mediates Aβ-related learning and memory deficits in a mouse model of Alzheimer's disease.Brain Res Bull. 2021 Dec;177:282-294. doi: 10.1016/j.brainresbull.2021.10.008. Epub 2021 Oct 20. Brain Res Bull. 2021. PMID: 34678444 Review.
Cited by
-
Non-coding RNAs: The Neuroinflammatory Regulators in Neurodegenerative Diseases.Front Neurol. 2022 Aug 12;13:929290. doi: 10.3389/fneur.2022.929290. eCollection 2022. Front Neurol. 2022. PMID: 36034298 Free PMC article. Review.
-
Molecular biomarkers for vascular cognitive impairment and dementia.Nat Rev Neurol. 2023 Dec;19(12):737-753. doi: 10.1038/s41582-023-00884-1. Epub 2023 Nov 13. Nat Rev Neurol. 2023. PMID: 37957261 Review.
-
The relationship between microRNAs and COVID-19 complications.Noncoding RNA Res. 2024 Aug 22;10:16-24. doi: 10.1016/j.ncrna.2024.08.007. eCollection 2025 Feb. Noncoding RNA Res. 2024. PMID: 39296641 Free PMC article. Review.
-
MicroRNAs: pioneering regulators in Alzheimer's disease pathogenesis, diagnosis, and therapy.Transl Psychiatry. 2024 Sep 10;14(1):367. doi: 10.1038/s41398-024-03075-8. Transl Psychiatry. 2024. PMID: 39256358 Free PMC article. Review.
-
Emerging Role of miR-21-5p in Neuron-Glia Dysregulation and Exosome Transfer Using Multiple Models of Alzheimer's Disease.Cells. 2022 Oct 26;11(21):3377. doi: 10.3390/cells11213377. Cells. 2022. PMID: 36359774 Free PMC article.
References
-
- Bar E, Barak B. Microglia roles in synaptic plasticity and myelination in homeostatic conditions and neurodevelopmental disorders. Glia. 2019;67:2125–41. - PubMed
-
- Liu HC, Zheng MH, Du YL, Wang L, Kuang F, Qin HY. et al. N9 microglial cells polarized by LPS and IL4 show differential responses to secondary environmental stimuli. Cell Immunol. 2012;278:84–90. - PubMed
-
- Wirz KT, Bossers K, Stargardt A, Kamphuis W, Swaab DF, Hol EM. et al. Cortical beta amyloid protein triggers an immune response, but no synaptic changes in the APPswe/PS1dE9 Alzheimer's disease mouse model. Neurobiol Aging. 2013;34:1328–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

