Downregulation of Macrophage-Specific Act-1 Intensifies Periodontitis and Alveolar Bone Loss Possibly via TNF/NF-κB Signaling
- PMID: 33748112
- PMCID: PMC7969798
- DOI: 10.3389/fcell.2021.628139
Downregulation of Macrophage-Specific Act-1 Intensifies Periodontitis and Alveolar Bone Loss Possibly via TNF/NF-κB Signaling
Abstract
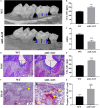
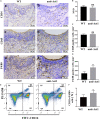
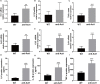
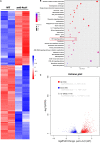
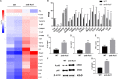
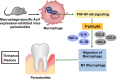
Periodontitis is a chronic inflammatory oral disease that affects almost half of the adult population. NF-κB activator 1 (Act1) is mainly expressed in immune cells, including macrophages, and modulates immune cells' function to regulate inflammation in inflammatory diseases. Macrophages play a vital role in the pathophysiology of periodontitis. However, the effect of macrophage-specific Act1 on periodontitis has not been investigated yet. This study aims to unravel the role of macrophage-specific Act1 on the pathophysiology of periodontitis. The expression of Act1 in healthy and periodontitis periodontal tissue was confirmed by immunohistochemistry. Macrophage-specific Act1 expression downregulated (anti-Act1) mice were developed by inserting anti-Act1 antisense oligonucleotides after the CD68 promoter of C57BL/6 mice. Ligature-induced periodontitis (LIP) was induced in anti-Act1 mice and wildtype mice. Micro-CT, histology, and TRAP staining analyzed the periodontal tissue status, alveolar bone loss, and osteoclast numbers. Immunohistochemistry, RT-qPCR, and ELISA analyzed the inflammatory cells infiltration, expression of inflammatory cytokines, and M1/M2 macrophage polarization. mRNA sequencing of in vitro bacterial lipopolysaccharide (LPS)-treated peritoneal macrophages analyzed the differentially expressed genes in anti-Act1 mice during inflammation. Anti-Act1 mice showed aggravated periodontitis and alveolar bone loss compared to wildtype. Periodontitis-affected periodontal tissue (PAPT) of anti-Act1 mice showed a higher degree of macrophage infiltration, and M1 macrophage polarization compared to wildtype. Levels of pro-inflammatory cytokines (IL-1β, IL-6, and TNFα), and macrophage activity-related factors (CCL2, CCL3, and CCL4) were robustly high in PAPT of anti-Act1 mice compared to wildtype. mRNA sequencing and KEGG analysis showed activated TNF/NF-κB signaling in LPS-treated macrophages from anti-Act1 mice. In vitro studies on LPS-treated peritoneal macrophages from anti-act1 mice showed a higher degree of cell migration and expression of inflammatory cytokines, macrophage activity-related factors, M1 macrophage-related factors, and TNF/NF-κB signaling related P-p65 protein. In conclusion, downregulation of macrophage-specific Act1 aggravated periodontitis, alveolar bone loss, macrophage infiltration, inflammation, and M1 macrophage polarization. Furthermore, LPS-treated macrophages from anti-Act1 mice activated TNF/NF-κB signaling. These results indicate the distinct role of macrophage-specific Act1 on the pathophysiology of periodontitis possibly via TNF/NF-κB signaling.
Keywords: NF-κB activator 1; alveolar bone loss; inflammation; macrophages; periodontitis.
Copyright © 2021 Pathak, Fang, Chen, Ye, Guo, Yan, Zha, Liang, Ke, Yang, Zhong, Wang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
SLIT2 Overexpression in Periodontitis Intensifies Inflammation and Alveolar Bone Loss, Possibly via the Activation of MAPK Pathway.Front Cell Dev Biol. 2020 Jul 14;8:593. doi: 10.3389/fcell.2020.00593. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32760720 Free PMC article.
-
Enoxaparin sodium bone cement plays an anti-inflammatory immunomodulatory role by inducing the polarization of M2 macrophages.J Orthop Surg Res. 2023 May 23;18(1):380. doi: 10.1186/s13018-023-03865-8. J Orthop Surg Res. 2023. PMID: 37221568 Free PMC article.
-
Hyperglycemia modulates M1/M2 macrophage polarization via reactive oxygen species overproduction in ligature-induced periodontitis.J Periodontal Res. 2021 Oct;56(5):991-1005. doi: 10.1111/jre.12912. Epub 2021 Jun 30. J Periodontal Res. 2021. PMID: 34190354
-
Novel inflammatory pathways in periodontitis.Adv Dent Res. 2014 May;26(1):23-9. doi: 10.1177/0022034514526240. Adv Dent Res. 2014. PMID: 24736701 Free PMC article. Review.
-
Polarization Profiles of T Lymphocytes and Macrophages Responses in Periodontitis.Adv Exp Med Biol. 2022;1373:195-208. doi: 10.1007/978-3-030-96881-6_10. Adv Exp Med Biol. 2022. PMID: 35612799 Review.
Cited by
-
Osteoclastogenesis Inhibitor and Antioxidant Properties of Konjac Glucomannan in a Periodontitis Mice Model: An In Vivo Study.Int J Dent. 2023 Oct 31;2023:7400421. doi: 10.1155/2023/7400421. eCollection 2023. Int J Dent. 2023. PMID: 37942469 Free PMC article.
-
Dicalcium silicate-induced mitochondrial dysfunction and autophagy-mediated macrophagic inflammation promotes osteogenic differentiation of BMSCs.Regen Biomater. 2021 Dec 13;9:rbab075. doi: 10.1093/rb/rbab075. eCollection 2022. Regen Biomater. 2021. PMID: 35480858 Free PMC article.
-
Glipizide Alleviates Periodontitis Pathogenicity via Inhibition of Angiogenesis, Osteoclastogenesis and M1/M2 Macrophage Ratio in Periodontal Tissue.Inflammation. 2023 Oct;46(5):1917-1931. doi: 10.1007/s10753-023-01850-1. Epub 2023 Jun 8. Inflammation. 2023. PMID: 37289398
-
Resveratrol Alleviates Diabetic Periodontitis-Induced Alveolar Osteocyte Ferroptosis Possibly via Regulation of SLC7A11/GPX4.Nutrients. 2023 Apr 28;15(9):2115. doi: 10.3390/nu15092115. Nutrients. 2023. PMID: 37432277 Free PMC article.
-
Macrophages and Bone Remodeling.J Bone Miner Res. 2023 Mar;38(3):359-369. doi: 10.1002/jbmr.4773. Epub 2023 Feb 3. J Bone Miner Res. 2023. PMID: 36651575 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

