Effects of Interleukin-1β in Glycinergic Transmission at the Central Amygdala
- PMID: 33746753
- PMCID: PMC7973117
- DOI: 10.3389/fphar.2021.613105
Effects of Interleukin-1β in Glycinergic Transmission at the Central Amygdala
Abstract
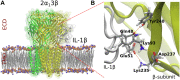
Interleukin-1β (IL-1β) is an important cytokine that modulates peripheral and central pain sensitization at the spinal level. Among its effects, it increases spinal cord excitability by reducing inhibitory Glycinergic and GABAergic neurotransmission. In the brain, IL-1β is released by glial cells in regions associated with pain processing during neuropathic pain. It also has important roles in neuroinflammation and in regulating NMDA receptor activity required for learning and memory. The modulation of glycine-mediated inhibitory activity via IL-1β may play a critical role in the perception of different levels of pain. The central nucleus of the amygdala (CeA) participates in receiving and processing pain information. Interestingly, this nucleus is enriched in the regulatory auxiliary glycine receptor (GlyR) β subunit (βGlyR); however, no studies have evaluated the effect of IL-1β on glycinergic neurotransmission in the brain. Hence, we hypothesized that IL-1β may modulate GlyR-mediated inhibitory activity via interactions with the βGlyR subunit. Our results show that the application of IL-1β (10 ng/ml) to CeA brain slices has a biphasic effect; transiently increases and then reduces sIPSC amplitude of CeA glycinergic currents. Additionally, we performed molecular docking, site-directed mutagenesis, and whole-cell voltage-clamp electrophysiological experiments in HEK cells transfected with GlyRs containing different GlyR subunits. These data indicate that IL-1β modulates GlyR activity by establishing hydrogen bonds with at least one key amino acid residue located in the back of the loop C at the ECD domain of the βGlyR subunit. The present results suggest that IL-1β in the CeA controls glycinergic neurotransmission, possibly via interactions with the βGlyR subunit. This effect could be relevant for understanding how IL-1β released by glia modulates central processing of pain, learning and memory, and is involved in neuroinflammation.
Keywords: auxiliary subunit; beta subunit; central amygdala (CeA); glycine receptors; interleukin-1β; neuroimmune communication.
Copyright © 2021 Solorza, Oliva, Castillo, Amestica, Maldifassi, López-Cortés, Barra, Stehberg, Piesche, Sáez-Briones, González, Arenas-Salinas and Mariqueo.
Conflict of interest statement
The authors hui declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Neuropathic Pain Induces Interleukin-1β Sensitive Bimodal Glycinergic Activity in the Central Amygdala.Int J Mol Sci. 2022 Jul 1;23(13):7356. doi: 10.3390/ijms23137356. Int J Mol Sci. 2022. PMID: 35806360 Free PMC article.
-
Heteromeric α/β glycine receptors regulate excitability in parvalbumin-expressing dorsal horn neurons through phasic and tonic glycinergic inhibition.J Physiol. 2017 Dec 1;595(23):7185-7202. doi: 10.1113/JP274926. Epub 2017 Oct 19. J Physiol. 2017. PMID: 28905384 Free PMC article.
-
IL-1 interacts with ethanol effects on GABAergic transmission in the mouse central amygdala.Front Pharmacol. 2015 Mar 19;6:49. doi: 10.3389/fphar.2015.00049. eCollection 2015. Front Pharmacol. 2015. PMID: 25852553 Free PMC article.
-
Native glycine receptor subtypes and their physiological roles.Neuropharmacology. 2009 Jan;56(1):303-9. doi: 10.1016/j.neuropharm.2008.07.034. Epub 2008 Aug 3. Neuropharmacology. 2009. PMID: 18721822 Review.
-
Molecular pharmacology of the glycine receptor chloride channel.Curr Pharm Des. 2007;13(23):2350-67. doi: 10.2174/138161207781368693. Curr Pharm Des. 2007. PMID: 17692006 Review.
Cited by
-
Neuropathic Pain Induces Interleukin-1β Sensitive Bimodal Glycinergic Activity in the Central Amygdala.Int J Mol Sci. 2022 Jul 1;23(13):7356. doi: 10.3390/ijms23137356. Int J Mol Sci. 2022. PMID: 35806360 Free PMC article.
-
Excitatory and inhibitory neuronal signaling in inflammatory and diabetic neuropathic pain.Mol Med. 2023 Apr 17;29(1):53. doi: 10.1186/s10020-023-00647-0. Mol Med. 2023. PMID: 37069517 Free PMC article. Review.
-
Recent Developments in Ion Channel and Ion-Related Signaling.Int J Mol Sci. 2023 Sep 22;24(19):14419. doi: 10.3390/ijms241914419. Int J Mol Sci. 2023. PMID: 37833868 Free PMC article.
-
Autonomic Nervous System Dysfunction Is Related to Chronic Prostatitis/Chronic Pelvic Pain Syndrome.World J Mens Health. 2024 Jan;42(1):1-28. doi: 10.5534/wjmh.220248. Epub 2023 Apr 19. World J Mens Health. 2024. PMID: 37118962 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

