Exercise, CaMKII, and type 2 diabetes
- PMID: 33746668
- PMCID: PMC7975583
- DOI: 10.17179/excli2020-3317
Exercise, CaMKII, and type 2 diabetes
Abstract
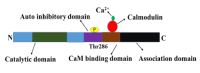
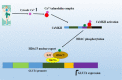
Individuals who exercise regularly are protected from type 2 diabetes and other metabolic syndromes, in part by enhanced gene transcription and induction of many signaling pathways crucial in correcting impaired metabolic pathways associated with a sedentary lifestyle. Exercise activates Calmodulin-dependent protein kinase (CaMK)II, resulting in increased mitochondrial oxidative capacity and glucose transport. CaMKII regulates many health beneficial cellular functions in individuals who exercise compared with those who do not exercise. The role of exercise in the regulation of carbohydrate, lipid metabolism, and insulin signaling pathways are explained at the onset. Followed by the role of exercise in the regulation of glucose transporter (GLUT)4 expression and mitochondrial biogenesis are explained. Next, the main functions of Calmodulin-dependent protein kinase and the mechanism to activate it are illustrated, finally, an overview of the role of CaMKII in regulating GLUT4 expression, mitochondrial biogenesis, and histone modification are discussed.
Keywords: CaMKII; GLUT4; Type 2 diabetes; exercise; insulin resistance; mitochondrial biogenesis.
Copyright © 2021 Joseph et al.
Figures

Similar articles
-
The role of CaMKII in regulating GLUT4 expression in skeletal muscle.Am J Physiol Endocrinol Metab. 2012 Aug 1;303(3):E322-31. doi: 10.1152/ajpendo.00091.2012. Epub 2012 Apr 10. Am J Physiol Endocrinol Metab. 2012. PMID: 22496345 Review.
-
Exercise increases hyper-acetylation of histones on the Cis-element of NRF-1 binding to the Mef2a promoter: Implications on type 2 diabetes.Biochem Biophys Res Commun. 2017 Apr 22;486(1):83-87. doi: 10.1016/j.bbrc.2017.03.002. Epub 2017 Mar 2. Biochem Biophys Res Commun. 2017. PMID: 28263745
-
Calmodulin dependent protein kinase II activation by exercise regulates saturated & unsaturated fatty acids and improves some metabolic syndrome markers.Life Sci. 2014 Aug 28;111(1-2):53-61. doi: 10.1016/j.lfs.2014.07.013. Epub 2014 Jul 19. Life Sci. 2014. PMID: 25046734
-
Role of CaMKII in the regulation of fatty acids and lipid metabolism.Diabetes Metab Syndr. 2021 Mar-Apr;15(2):589-594. doi: 10.1016/j.dsx.2021.02.037. Epub 2021 Mar 3. Diabetes Metab Syndr. 2021. PMID: 33714133 Review.
-
Mitochondrial biogenesis: pharmacological approaches.Curr Pharm Des. 2014;20(35):5507-9. doi: 10.2174/138161282035140911142118. Curr Pharm Des. 2014. PMID: 24606795
Cited by
-
Exercise, Mitohormesis, and Mitochondrial ORF of the 12S rRNA Type-C (MOTS-c).Diabetes Metab J. 2022 May;46(3):402-413. doi: 10.4093/dmj.2022.0092. Epub 2022 May 25. Diabetes Metab J. 2022. PMID: 35656563 Free PMC article. Review.
-
Harnessing Mitochondrial Stress for Health and Disease: Opportunities and Challenges.Biology (Basel). 2024 May 29;13(6):394. doi: 10.3390/biology13060394. Biology (Basel). 2024. PMID: 38927274 Free PMC article. Review.
-
Impact of Exercise and Aging on Mitochondrial Homeostasis in Skeletal Muscle: Roles of ROS and Epigenetics.Cells. 2022 Jun 30;11(13):2086. doi: 10.3390/cells11132086. Cells. 2022. PMID: 35805170 Free PMC article. Review.
References
-
- Araki E, Lipes MA, Pattti ME, Khan CR. Alternative pathway of insulin signaling in mice with targeted disruption of the IRS- gene. Nature. 1994;372:186–190. - PubMed
-
- Baron AD, Brechtel G, Wallace P, Edelman SV. Rates and tissue sites of non-insulin and insulin mediated glucose uptake in humans. Am J Physiol. 1988;255:E769–E774. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
