Protein oxidation - Formation mechanisms, detection and relevance as biomarkers in human diseases
- PMID: 33744200
- PMCID: PMC8113053
- DOI: 10.1016/j.redox.2021.101901
Protein oxidation - Formation mechanisms, detection and relevance as biomarkers in human diseases
Abstract
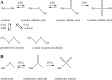
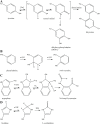
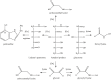
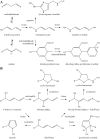
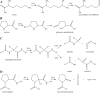
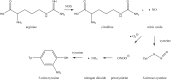
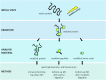
Generation of reactive oxygen species and related oxidants is an inevitable consequence of life. Proteins are major targets for oxidation reactions, because of their rapid reaction rates with oxidants and their high abundance in cells, extracellular tissues, and body fluids. Additionally, oxidative stress is able to degrade lipids and carbohydrates to highly reactive intermediates, which eventually attack proteins at various functional sites. Consequently, a wide variety of distinct posttranslational protein modifications is formed by protein oxidation, glycoxidation, and lipoxidation. Reversible modifications are relevant in physiological processes and constitute signaling mechanisms ("redox signaling"), while non-reversible modifications may contribute to pathological situations and several diseases. A rising number of publications provide evidence for their involvement in the onset and progression of diseases as well as aging processes. Certain protein oxidation products are chemically stable and formed in large quantity, which makes them promising candidates to become biomarkers of oxidative damage. Moreover, progress in the development of detection and quantification methods facilitates analysis time and effort and contributes to their future applicability in clinical routine. The present review outlines the most important classes and selected examples of oxidative protein modifications, elucidates the chemistry beyond their formation and discusses available methods for detection and analysis. Furthermore, the relevance and potential of protein modifications as biomarkers in the context of disease and aging is summarized.
Keywords: Aging; Biomarker; Oxidative stress; Protein modification; Protein oxidation.
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Figures

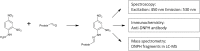
Similar articles
-
Detection, identification, and quantification of oxidative protein modifications.J Biol Chem. 2019 Dec 20;294(51):19683-19708. doi: 10.1074/jbc.REV119.006217. Epub 2019 Oct 31. J Biol Chem. 2019. PMID: 31672919 Free PMC article. Review.
-
Oxidative stress and protein aggregation during biological aging.Exp Gerontol. 2001 Sep;36(9):1539-50. doi: 10.1016/s0531-5565(01)00139-5. Exp Gerontol. 2001. PMID: 11525876 Review.
-
Detection and localization of markers of oxidative stress by in situ methods: application in the study of Alzheimer disease.Methods Mol Biol. 2010;610:419-34. doi: 10.1007/978-1-60327-029-8_25. Methods Mol Biol. 2010. PMID: 20013193 Free PMC article.
-
Lipoxidation in cardiovascular diseases.Redox Biol. 2019 May;23:101119. doi: 10.1016/j.redox.2019.101119. Epub 2019 Feb 25. Redox Biol. 2019. PMID: 30833142 Free PMC article. Review.
-
Biomarkers of protein oxidation in human disease.Curr Mol Med. 2012 Jul 1;12(6):681-97. doi: 10.2174/156652412800792543. Curr Mol Med. 2012. PMID: 22292436 Review.
Cited by
-
The Role of Selenitetriglycerides in Enhancing Antioxidant Defense Mechanisms in Peripartum Holstein-Friesian Cows.Animals (Basel). 2024 Feb 13;14(4):610. doi: 10.3390/ani14040610. Animals (Basel). 2024. PMID: 38396578 Free PMC article.
-
Evaluating β-cryptoxanthin antioxidant properties against ROS-induced macromolecular damages and determining its photo-stability and in-vitro SPF.World J Microbiol Biotechnol. 2023 Sep 16;39(11):310. doi: 10.1007/s11274-023-03747-5. World J Microbiol Biotechnol. 2023. PMID: 37715879
-
Oxidative Stress Biomarkers in Urine of Metal Carpentry Workers Can Be Diagnostic for Occupational Exposure to Low Level of Welding Fumes from Associated Metals.Cancers (Basel). 2021 Jun 24;13(13):3167. doi: 10.3390/cancers13133167. Cancers (Basel). 2021. PMID: 34202906 Free PMC article.
-
Skin senescence-from basic research to clinical practice.Front Med (Lausanne). 2024 Oct 18;11:1484345. doi: 10.3389/fmed.2024.1484345. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39493718 Free PMC article. Review.
-
The RPN12a proteasome subunit is essential for the multiple hormonal homeostasis controlling the progression of leaf senescence.Commun Biol. 2022 Sep 30;5(1):1043. doi: 10.1038/s42003-022-03998-2. Commun Biol. 2022. PMID: 36180574 Free PMC article.
References
-
- Sies H. Strategies of antioxidant defense. Eur. J. Biochem. 1993;215:213–219. - PubMed
-
- Hughes M.N., Nicklin H.G. A possible role for the species peroxonitrite in nitrification. Biochim. Biophys. Acta. 1970;222:660–661. - PubMed
-
- Kregel K.C., Zhang H.J. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R18–R36. - PubMed
-
- Sies H., Berndt C., Jones D.P. Oxidative stress. Annu. Rev. Biochem. 2017;86:715–748. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

