Induced Pluripotent Stem Cells (iPSCs) in Vascular Research: from Two- to Three-Dimensional Organoids
- PMID: 33738695
- PMCID: PMC7972819
- DOI: 10.1007/s12015-021-10149-3
Induced Pluripotent Stem Cells (iPSCs) in Vascular Research: from Two- to Three-Dimensional Organoids
Abstract
Stem cell technology has been around for almost 30 years and in that time has grown into an enormous field. The stem cell technique progressed from the first successful isolation of mammalian embryonic stem cells (ESCs) in the 1990s, to the production of human induced-pluripotent stem cells (iPSCs) in the early 2000s, to finally culminate in the differentiation of pluripotent cells into highly specialized cell types, such as neurons, endothelial cells (ECs), cardiomyocytes, fibroblasts, and lung and intestinal cells, in the last decades. In recent times, we have attained a new height in stem cell research whereby we can produce 3D organoids derived from stem cells that more accurately mimic the in vivo environment. This review summarizes the development of stem cell research in the context of vascular research ranging from differentiation techniques of ECs and smooth muscle cells (SMCs) to the generation of vascularized 3D organoids. Furthermore, the different techniques are critically reviewed, and future applications of current 3D models are reported.
Keywords: Differentiation; Endothelial cells; Induced pluripotent stem cells; Organoids; Smooth muscle cells; Vasculature.
© 2021. The Author(s).
Conflict of interest statement
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Figures
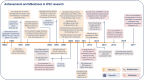
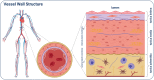
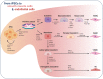

Similar articles
-
Dynamic Characterization of Structural, Molecular, and Electrophysiological Phenotypes of Human-Induced Pluripotent Stem Cell-Derived Cerebral Organoids, and Comparison with Fetal and Adult Gene Profiles.Cells. 2020 May 23;9(5):1301. doi: 10.3390/cells9051301. Cells. 2020. PMID: 32456176 Free PMC article.
-
3D brain Organoids derived from pluripotent stem cells: promising experimental models for brain development and neurodegenerative disorders.J Biomed Sci. 2017 Aug 20;24(1):59. doi: 10.1186/s12929-017-0362-8. J Biomed Sci. 2017. PMID: 28822354 Free PMC article. Review.
-
Organoids in domestic animals: with which stem cells?Vet Res. 2021 Mar 4;52(1):38. doi: 10.1186/s13567-021-00911-3. Vet Res. 2021. PMID: 33663614 Free PMC article. Review.
-
Generation of human vascularized brain organoids.Neuroreport. 2018 May 2;29(7):588-593. doi: 10.1097/WNR.0000000000001014. Neuroreport. 2018. PMID: 29570159 Free PMC article.
-
Development of intestinal organoids as tissue surrogates: cell composition and the epigenetic control of differentiation.Mol Carcinog. 2015 Mar;54(3):189-202. doi: 10.1002/mc.22089. Epub 2013 Sep 21. Mol Carcinog. 2015. PMID: 24115167
Cited by
-
Merits and challenges of iPSC-derived organoids for clinical applications.Front Cell Dev Biol. 2023 May 26;11:1188905. doi: 10.3389/fcell.2023.1188905. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37305682 Free PMC article. Review.
-
Anticonstriction Effect of MCA in Rats by Danggui Buxue Decoction.Front Pharmacol. 2021 Nov 8;12:749915. doi: 10.3389/fphar.2021.749915. eCollection 2021. Front Pharmacol. 2021. PMID: 34867357 Free PMC article.
-
From the Dish to the Real World: Modeling Interactions between the Gut and Microorganisms in Gut Organoids by Tailoring the Gut Milieu.Int J Stem Cells. 2022 Feb 28;15(1):70-84. doi: 10.15283/ijsc21243. Int J Stem Cells. 2022. PMID: 35220293 Free PMC article. Review.
-
From bedside to the bench: patient-specific hiPSC-EC models uncover endothelial dysfunction in genetic cardiomyopathies.Front Physiol. 2023 Jul 19;14:1237101. doi: 10.3389/fphys.2023.1237101. eCollection 2023. Front Physiol. 2023. PMID: 37538375 Free PMC article. Review.
-
Understanding genomic medicine for thoracic aortic disease through the lens of induced pluripotent stem cells.Front Cardiovasc Med. 2024 Feb 19;11:1349548. doi: 10.3389/fcvm.2024.1349548. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38440211 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

