The Third Man: DNA sensing as espionage in pulmonary vascular health and disease
- PMID: 33738095
- PMCID: PMC7934053
- DOI: 10.1177/2045894021996574
The Third Man: DNA sensing as espionage in pulmonary vascular health and disease
Abstract
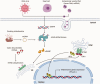
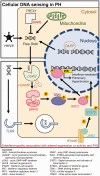
For as long as nucleic acids have been utilized to vertically and horizontally transfer genetic material, living organisms have had to develop methods of recognizing cytosolic DNA as either pathogenic (microbial invasion) or physiologic (mitosis and cellular proliferation). Derangement in key signaling molecules involved in these pathways of DNA sensing result in a family of diseases labeled interferonopathies. An interferonopathy, characterized by constitutive expression of type I interferons, ultimately manifests as severe autoimmune disease at a young age. Afflicted patients present with a constellation of immune-mediated conditions, including primary lung manifestations such as pulmonary fibrosis and pulmonary hypertension. The latter condition is especially interesting in light of the known role that DNA damage plays in a variety of types of inherited and induced pulmonary hypertension, with free DNA detection elevated in the circulation of affected individuals. While little is known regarding the role of cytosolic DNA sensing in development of pulmonary vascular disease, exciting new research in the related fields of immunology and oncology potentially sheds light on future areas of fruitful exploration. As such, the goal of this review is to summarize the state of the field of nucleic acid sensing, extrapolating common shared pathways that parallel our knowledge of pulmonary hypertension, in a molecular and cell-specific manner. Principles of DNA sensing related to known pulmonary injury inducing stimuli are also evaluated, in addition to potential therapeutic targets. Finally, future directions in pulmonary hypertension research and treatments will be briefly discussed.
Keywords: cyclic GMP-AMP synthase (cGAS); interferonopathy; mitochondrial DNA (mtDNA); stimulator of interferon genes (STING); toll-like receptor 9 (TLR9).
© The Author(s) 2021.
Figures
Similar articles
-
The cGAS-STING signaling in cardiovascular and metabolic diseases: Future novel target option for pharmacotherapy.Acta Pharm Sin B. 2022 Jan;12(1):50-75. doi: 10.1016/j.apsb.2021.05.011. Epub 2021 May 20. Acta Pharm Sin B. 2022. PMID: 35127372 Free PMC article. Review.
-
Triggering of the cGAS-STING Pathway in Human Plasmacytoid Dendritic Cells Inhibits TLR9-Mediated IFN Production.J Immunol. 2020 Jul 1;205(1):223-236. doi: 10.4049/jimmunol.1800933. Epub 2020 May 29. J Immunol. 2020. PMID: 32471881 Free PMC article.
-
The Trinity of cGAS, TLR9, and ALRs Guardians of the Cellular Galaxy Against Host-Derived Self-DNA.Front Immunol. 2021 Feb 11;11:624597. doi: 10.3389/fimmu.2020.624597. eCollection 2020. Front Immunol. 2021. PMID: 33643304 Free PMC article. Review.
-
The role of cGAS-STING signalling in liver diseases.JHEP Rep. 2021 Jun 24;3(5):100324. doi: 10.1016/j.jhepr.2021.100324. eCollection 2021 Oct. JHEP Rep. 2021. PMID: 34381984 Free PMC article. Review.
-
Human plasmacytoid dentritic cells elicit a Type I Interferon response by sensing DNA via the cGAS-STING signaling pathway.Eur J Immunol. 2016 Jul;46(7):1615-21. doi: 10.1002/eji.201546113. Epub 2016 May 27. Eur J Immunol. 2016. PMID: 27125983 Free PMC article.
Cited by
-
Non-Interferon-Dependent Role of STING Signaling in Pulmonary Hypertension.Arterioscler Thromb Vasc Biol. 2024 Jan;44(1):124-142. doi: 10.1161/ATVBAHA.123.320121. Epub 2023 Nov 9. Arterioscler Thromb Vasc Biol. 2024. PMID: 37942608 Free PMC article.
-
Experimental congenital diaphragmatic hernia features an alteration of DNA sensing targets cGAS and STING.Pediatr Res. 2024 May 30. doi: 10.1038/s41390-024-03277-2. Online ahead of print. Pediatr Res. 2024. PMID: 38816442
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

