Hnf1b haploinsufficiency differentially affects developmental target genes in a new renal cysts and diabetes mouse model
- PMID: 33737325
- PMCID: PMC8126479
- DOI: 10.1242/dmm.047498
Hnf1b haploinsufficiency differentially affects developmental target genes in a new renal cysts and diabetes mouse model
Abstract
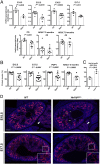
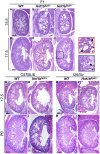
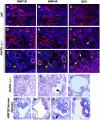
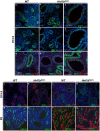
Heterozygous mutations in HNF1B cause the complex syndrome renal cysts and diabetes (RCAD), characterized by developmental abnormalities of the kidneys, genital tracts and pancreas, and a variety of renal, pancreas and liver dysfunctions. The pathogenesis underlying this syndrome remains unclear as mice with heterozygous null mutations have no phenotype, while constitutive/conditional Hnf1b ablation leads to more severe phenotypes. We generated a novel mouse model carrying an identified human mutation at the intron-2 splice donor site. Unlike heterozygous mice previously characterized, mice heterozygous for the splicing mutation exhibited decreased HNF1B protein levels and bilateral renal cysts from embryonic day 15, originated from glomeruli, early proximal tubules (PTs) and intermediate nephron segments, concurrently with delayed PT differentiation, hydronephrosis and rare genital tract anomalies. Consistently, mRNA sequencing showed that most downregulated genes in embryonic kidneys were primarily expressed in early PTs and the loop of Henle and involved in ion/drug transport, organic acid and lipid metabolic processes, while the expression of previously identified targets upon Hnf1b ablation, including cystic disease genes, was weakly or not affected. Postnatal analyses revealed renal abnormalities, ranging from glomerular cysts to hydronephrosis and, rarely, multicystic dysplasia. Urinary proteomics uncovered a particular profile predictive of progressive decline in kidney function and fibrosis, and displayed common features with a recently reported urine proteome in an RCAD pediatric cohort. Altogether, our results show that reduced HNF1B levels lead to developmental disease phenotypes associated with the deregulation of a subset of HNF1B targets. They further suggest that this model represents a unique clinical/pathological viable model of the RCAD disease.
Keywords: Gene dosage; Glomerular and proximal tubule cysts; HNF1B transcription factor; Mouse models; RCAD syndrome; Transcriptomics.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


Similar articles
-
Insights into the etiology and physiopathology of MODY5/HNF1B pancreatic phenotype with a mouse model of the human disease.J Pathol. 2021 May;254(1):31-45. doi: 10.1002/path.5629. Epub 2021 Mar 18. J Pathol. 2021. PMID: 33527355 Free PMC article.
-
A novel mutation of the HNF1B gene associated with hypoplastic glomerulocystic kidney disease and neonatal renal failure: a case report and mutation update.Medicine (Baltimore). 2015 Feb;94(7):e469. doi: 10.1097/MD.0000000000000469. Medicine (Baltimore). 2015. PMID: 25700310 Free PMC article.
-
A novel HNF1B mutation p.R177Q in autosomal dominant tubulointerstitial kidney disease and maturity-onset diabetes of the young type 5: A pedigree-based case report.Medicine (Baltimore). 2020 Jul 31;99(31):e21438. doi: 10.1097/MD.0000000000021438. Medicine (Baltimore). 2020. PMID: 32756155 Free PMC article.
-
Hepatic phenotypes of HNF1B gene mutations: a case of neonatal cholestasis requiring portoenterostomy and literature review.World J Gastroenterol. 2015 Feb 28;21(8):2550-7. doi: 10.3748/wjg.v21.i8.2550. World J Gastroenterol. 2015. PMID: 25741167 Free PMC article. Review.
-
The role of hepatocyte nuclear factor 1β in disease and development.Diabetes Obes Metab. 2016 Sep;18 Suppl 1:23-32. doi: 10.1111/dom.12715. Diabetes Obes Metab. 2016. PMID: 27615128 Review.
Cited by
-
HNF1B Transcription Factor: Key Regulator in Renal Physiology and Pathogenesis.Int J Mol Sci. 2024 Oct 2;25(19):10609. doi: 10.3390/ijms251910609. Int J Mol Sci. 2024. PMID: 39408938 Free PMC article. Review.
-
The role of noncoding RNAs in pancreatic birth defects.Birth Defects Res. 2023 Nov 15;115(19):1785-1808. doi: 10.1002/bdr2.2178. Epub 2023 Apr 17. Birth Defects Res. 2023. PMID: 37066622 Free PMC article. Review.
-
Sexually dimorphic renal expression of mouse Klotho is directed by a kidney-specific distal enhancer responsive to HNF1b.Commun Biol. 2024 Sep 14;7(1):1142. doi: 10.1038/s42003-024-06855-6. Commun Biol. 2024. PMID: 39277686 Free PMC article.
-
Hnf1b renal expression directed by a distal enhancer responsive to Pax8.Sci Rep. 2022 Nov 19;12(1):19921. doi: 10.1038/s41598-022-21171-x. Sci Rep. 2022. PMID: 36402859 Free PMC article.
-
Precocious puberty or growth hormone deficiency as initial presentation in Mayer-Rokitansky-kuster-Hauser syndrome: a clinical report of 5 cases.BMC Pediatr. 2022 Jul 14;22(1):418. doi: 10.1186/s12887-022-03474-0. BMC Pediatr. 2022. PMID: 35836205 Free PMC article.
References
-
- Aboudehen, K., Noureddine, L., Cobo-Stark, P., Avdulov, S., Farahani, S., Gearhart, M. D., Bichet, D. G., Pontoglio, M., Patel, V. and Igarashi, P. (2017). Hepatocyte Nuclear Factor-1beta regulates urinary concentration and response to hypertonicity. J. Am. Soc. Nephrol. 28, 2887-2900. 10.1681/ASN.2016101095 - DOI - PMC - PubMed
-
- Adalat, S., Woolf, A. S., Johnstone, K. A., Wirsing, A., Harries, L. W., Long, D. A., Hennekam, R. C., Ledermann, S. E., Rees, L., van't Hoff, W.et al. (2009). HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. J. Am. Soc. Nephrol. 20, 1123-1131. 10.1681/ASN.2008060633 - DOI - PMC - PubMed
-
- Adalat, S., Hayes, W. N., Bryant, W. A., Booth, J., Woolf, A. S., Kleta, R., Subtil, S., Clissold, R., Colclough, K., Ellard, S.et al. (2019). HNF1B mutations are associated with a Gitelman-like tubulopathy that develops during childhood. Kidney Int. Rep. 4, 1304-1311. 10.1016/j.ekir.2019.05.019 - DOI - PMC - PubMed
-
- Alvelos, M. I., Rodrigues, M., Lobo, L., Medeira, A., Sousa, A. B., Simão, C. and Lemos, M. C. (2015). A novel mutation of the HNF1B gene associated with hypoplastic glomerulocystic kidney disease and neonatal renal failure: a case report and mutation update. Medicine (Baltim.) 94, e469. 10.1097/MD.0000000000000469 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

