Low-Dose Tamoxifen for Mammographic Density Reduction: A Randomized Controlled Trial
- PMID: 33734864
- PMCID: PMC8189632
- DOI: 10.1200/JCO.20.02598
Low-Dose Tamoxifen for Mammographic Density Reduction: A Randomized Controlled Trial
Abstract
Purpose: Tamoxifen prevents breast cancer in high-risk women and reduces mortality in the adjuvant setting. Mammographic density change is a proxy for tamoxifen therapy response. We tested whether lower doses of tamoxifen were noninferior to reduce mammographic density and associated with fewer symptoms.
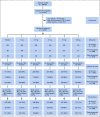
Patients and methods: Women, 40-74 years of age, participating in the Swedish mammography screening program were invited to the 6-month double-blind six-arm randomized placebo-controlled noninferiority dose-determination KARISMA phase II trial stratified by menopausal status (EudraCT 2016-000882-22). In all, 1,439 women were accrued with 1,230 participants accessible for intention-to-treat analysis. The primary outcome was proportion of women treated with placebo, 1, 2.5, 5, and 10 mg whose mammographic density decreased at least as much as the median reduction in the 20 mg arm. The noninferior margin was 17%. Secondary outcome was reduction of symptoms. Post hoc analyses were performed by menopausal status. Per-protocol population and full population were analyzed in sensitivity analysis.
Results: The 1,439 participants, 566 and 873 pre- and postmenopausal women, respectively, were recruited between October 1, 2016, and September 30, 2019. The participants had noninferior mammographic density reduction following 2.5, 5, and 10 mg tamoxifen compared with the median 10.1% decrease observed in the 20 mg group, a reduction confined to premenopausal women. Severe vasomotor symptoms (hot flashes, cold sweats, and night sweats) were reduced by approximately 50% in the 2.5, 5, and 10 mg groups compared with the 20 mg group.
Conclusion: Premenopausal women showed noninferior magnitude of breast density decrease at 2.5 mg of tamoxifen, but fewer side effects compared with the standard dose of 20 mg. Future studies should test whether 2.5 mg of tamoxifen reduces the risk of primary breast cancer.
Conflict of interest statement
Figures

Comment in
-
Regarding the Appropriate Target and Duration of Chemoprevention in Breast Cancer.J Clin Oncol. 2021 Sep 10;39(26):2965-2966. doi: 10.1200/JCO.21.01060. Epub 2021 Jun 14. J Clin Oncol. 2021. PMID: 34125580 No abstract available.
-
Reply to T. Suemasu et al.J Clin Oncol. 2021 Sep 10;39(26):2966-2968. doi: 10.1200/JCO.21.01166. Epub 2021 Jun 14. J Clin Oncol. 2021. PMID: 34125585 No abstract available.
Similar articles
-
Baseline breast tissue characteristics determine the effect of tamoxifen on mammographic density change.Int J Cancer. 2024 Jul 15;155(2):339-351. doi: 10.1002/ijc.34939. Epub 2024 Mar 30. Int J Cancer. 2024. PMID: 38554131 Clinical Trial.
-
Randomized double-blind 2 x 2 trial of low-dose tamoxifen and fenretinide for breast cancer prevention in high-risk premenopausal women.J Clin Oncol. 2009 Aug 10;27(23):3749-56. doi: 10.1200/JCO.2008.19.3797. Epub 2009 Jul 13. J Clin Oncol. 2009. PMID: 19597031 Free PMC article. Clinical Trial.
-
Effects of tamoxifen on normal breast tissue histological composition: Results from a randomised six-arm placebo-controlled trial in healthy women.Int J Cancer. 2023 Jun 1;152(11):2362-2372. doi: 10.1002/ijc.34430. Epub 2023 Jan 22. Int J Cancer. 2023. PMID: 36637153 Clinical Trial.
-
Mammographic density: a potential monitoring biomarker for adjuvant and preventative breast cancer endocrine therapies.Oncotarget. 2017 Jan 17;8(3):5578-5591. doi: 10.18632/oncotarget.13484. Oncotarget. 2017. PMID: 27894075 Free PMC article. Review.
-
Anastrozole as adjuvant therapy for early-stage breast cancer: implications of the ATAC trial.Clin Breast Cancer. 2003 Apr;4 Suppl 1:S42-8. doi: 10.3816/cbc.2003.s.014. Clin Breast Cancer. 2003. PMID: 12756078 Review.
Cited by
-
Tamoxifen Dose De-Escalation: An Effective Strategy for Reducing Adverse Effects?Drugs. 2024 Apr;84(4):385-401. doi: 10.1007/s40265-024-02010-x. Epub 2024 Mar 14. Drugs. 2024. PMID: 38480629 Free PMC article. Review.
-
Preliminary results using a kit to measure tamoxifen and metabolites concentrations in capillary blood samples from women with breast cancer.Sci Rep. 2022 Jan 31;12(1):1643. doi: 10.1038/s41598-022-05443-0. Sci Rep. 2022. PMID: 35102224 Free PMC article. Clinical Trial.
-
The impact of endoxifen-guided tamoxifen dose reductions on endocrine side-effects in patients with primary breast cancer.ESMO Open. 2023 Feb;8(1):100786. doi: 10.1016/j.esmoop.2023.100786. Epub 2023 Feb 6. ESMO Open. 2023. PMID: 36753991 Free PMC article.
-
FGF/FGFR1 system in paired breast tumor-adjacent and tumor tissues, associations with mammographic breast density and tumor characteristics.Front Oncol. 2023 Jul 20;13:1230821. doi: 10.3389/fonc.2023.1230821. eCollection 2023. Front Oncol. 2023. PMID: 37546410 Free PMC article.
-
Use of Low-Dose Tamoxifen to Increase Mammographic Screening Sensitivity in Premenopausal Women.Cancers (Basel). 2021 Jan 15;13(2):302. doi: 10.3390/cancers13020302. Cancers (Basel). 2021. PMID: 33467653 Free PMC article.
References
-
- American Cancer Society : Breast Cancer Facts & Figures 2019-2020. Atlanta, GA, American Cancer Society, 2019
-
- Fisher B Costantino JP Wickerham DL, et al. : Tamoxifen for the prevention of breast cancer: Current status of the national surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst 97:1652-1662, 2005 - PubMed
-
- Powles TJ Ashley S Tidy A, et al. : Twenty year follow-up of the Royal Marsden randomized, double blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst 99:283-290, 2007 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

