Plasma Cytokine Profiling to Predict Steroid Resistance in Pediatric Nephrotic Syndrome
- PMID: 33732993
- PMCID: PMC7938200
- DOI: 10.1016/j.ekir.2020.12.027
Plasma Cytokine Profiling to Predict Steroid Resistance in Pediatric Nephrotic Syndrome
Abstract
Introduction: Glucocorticoids (GCs) are the primary treatment for nephrotic syndrome (NS), although ∼10% to 20% of children develop steroid-resistant NS (SRNS). Unfortunately, there are no validated biomarkers able to predict SRNS at initial disease presentation. We hypothesized that a plasma cytokine panel could predict SRNS at disease presentation, and identify potential pathways regulating SRNS pathogenesis.
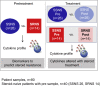
Methods: Paired plasma samples were collected from 26 children with steroid-sensitive NS (SSNS) and 14 with SRNS at NS presentation and after ∼7 weeks of GC therapy, when SSNS versus SRNS was clinically determined. Plasma cytokine profiling was performed with a panel of 27 cytokines.
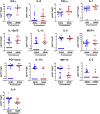
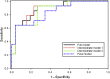
Results: We identified 13 cytokines significantly different in Pretreatment SSNS versus SRNS samples. Statistical modeling identified a cytokine panel (interleukin [IL]-7, IL-9, monocyte chemoattractant protein-1 [MCP-1]) able to discriminate between SSNS and SRNS at disease presentation (receiver operating characteristic [ROC] value = 0.846; sensitivity = 0.643; specificity = 0.846). Furthermore, GC treatment resulted in significant decreases in plasma interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), IL-7, IL-13, and IL-5 in both SSNS and SRNS patients.
Conclusions: These studies suggest that initial GC treatment of NS reduces the plasma cytokines secreted by both CD4+ TH1 cells and TH2 cells, as well as CD8+ T cells. Importantly, a panel of 3 cytokines (IL-7, IL-9, and MCP-1) was able to predict SRNS prior to GC treatment at disease presentation. Although these findings will benefit from validation in a larger cohort, the ability to identify SRNS at disease presentation could greatly benefit patients by enabling both avoidance of unnecessary GC-induced toxicity and earlier transition to more effective alternative treatments.
Keywords: Steroids; biomarkers; cytokines; glucocorticoids; steroid-resistant nephrotic syndrome; steroid-sensitive nephrotic syndrome.
© 2021 International Society of Nephrology. Published by Elsevier Inc.
Figures

Similar articles
-
Predicting and Defining Steroid Resistance in Pediatric Nephrotic Syndrome Using Plasma Proteomics.Kidney Int Rep. 2019 Sep 19;5(1):66-80. doi: 10.1016/j.ekir.2019.09.009. eCollection 2020 Jan. Kidney Int Rep. 2019. PMID: 31922062 Free PMC article.
-
Regulatory and effector T cells changes in remission and resistant state of childhood nephrotic syndrome.Indian J Nephrol. 2014 Nov;24(6):349-55. doi: 10.4103/0971-4065.132992. Indian J Nephrol. 2014. PMID: 25484527 Free PMC article.
-
Multiomics Analysis of Plasma Proteomics and Metabolomics of Steroid Resistance in Childhood Nephrotic Syndrome Using a "Patient-Specific" Approach.Kidney Int Rep. 2023 Mar 23;8(6):1239-1254. doi: 10.1016/j.ekir.2023.03.015. eCollection 2023 Jun. Kidney Int Rep. 2023. PMID: 37284673 Free PMC article.
-
The Role of Cytokines in Nephrotic Syndrome.Mediators Inflamm. 2022 Feb 9;2022:6499668. doi: 10.1155/2022/6499668. eCollection 2022. Mediators Inflamm. 2022. PMID: 35185384 Free PMC article. Review.
-
Genetics of childhood steroid-sensitive nephrotic syndrome.Pediatr Nephrol. 2017 Sep;32(9):1481-1488. doi: 10.1007/s00467-016-3456-8. Epub 2016 Jul 29. Pediatr Nephrol. 2017. PMID: 27470160 Free PMC article. Review.
Cited by
-
Effect of Huaiqihuang Granules Combined with Comprehensive Nursing on Children with Primary Nephrotic Syndrome.J Healthc Eng. 2022 Jan 15;2022:3279503. doi: 10.1155/2022/3279503. eCollection 2022. J Healthc Eng. 2022. PMID: 35075385 Free PMC article. Clinical Trial.
-
Sulfatase 2 Is Associated with Steroid Resistance in Childhood Nephrotic Syndrome.J Clin Med. 2021 Feb 2;10(3):523. doi: 10.3390/jcm10030523. J Clin Med. 2021. PMID: 33540508 Free PMC article.
-
Later Response to Corticosteroids in Adults With Primary Focal Segmental Glomerular Sclerosis Is Associated With Favorable Outcomes.Kidney Int Rep. 2021 Oct 29;7(1):87-98. doi: 10.1016/j.ekir.2021.10.016. eCollection 2022 Jan. Kidney Int Rep. 2021. PMID: 35005317 Free PMC article.
-
Swollen Feet: Considering the Paradoxical Roles of Interleukins in Nephrotic Syndrome.Biomedicines. 2024 Mar 26;12(4):738. doi: 10.3390/biomedicines12040738. Biomedicines. 2024. PMID: 38672094 Free PMC article. Review.
-
Podocyte-targeted therapies - progress and future directions.Nat Rev Nephrol. 2024 Oct;20(10):643-658. doi: 10.1038/s41581-024-00843-z. Epub 2024 May 9. Nat Rev Nephrol. 2024. PMID: 38724717 Review.
References
-
- Shalhoub R.J. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet. 1974;2:556–560. - PubMed
-
- Kurts C., Panzer U., Anders H.J. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13:738–753. - PubMed
-
- Pereira Wde F., Brito-Melo G.E., Guimaraes F.T. The role of the immune system in idiopathic nephrotic syndrome: a review of clinical and experimental studies. Inflamm Res. 2014;63:1–12. - PubMed
-
- Cho M.H., Lee H.S., Choe B.H. Interleukin-8 and tumor necrosis factor-alpha are increased in minimal change disease but do not alter albumin permeability. Am J Nephrol. 2003;23:260–266. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

