Pemafibrate suppresses oxidative stress and apoptosis under cardiomyocyte ischemia-reperfusion injury in type 1 diabetes mellitus
- PMID: 33732304
- PMCID: PMC7903427
- DOI: 10.3892/etm.2021.9762
Pemafibrate suppresses oxidative stress and apoptosis under cardiomyocyte ischemia-reperfusion injury in type 1 diabetes mellitus
Abstract
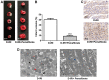
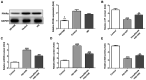
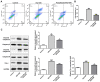
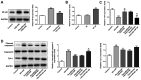
Diabetes mellitus accelerates the hyperglycemia susceptibility-induced injury to cardiac cells. The activation of peroxisome proliferator-activated receptor α (PPARα) decreases ischemia-reperfusion (IR) injury in animals without diabetes. Therefore, the present study hypothesized that pemafibrate may exert a protective effect on the myocardium in vivo and in vitro. A type 1 diabetes mellitus (T1DM) rat model and H9c2 cells exposed to high glucose under hypoxia and reoxygenation treatments were used in the present study. The rat model and the cells were subsequently treated with pemafibrate. In the T1DM rat model, pemafibrate enhanced the expression of PPARα in the diabetic-myocardial ischemia-reperfusion injury (D-IRI) group compared with the D-IRI group. The infarct size in the D-IRI group was reduced following pemafibrate treatment relative to the untreated group. The disruption of the mitochondrial structure and myofibrils in the D-IRI group was partially recovered by pemafibrate. In addition, to evaluate the mechanism of action of pemafibrate in the treatment of diabetic myocardial IR injury, an in vitro model was established. PPARα protein expression levels were reduced in the high glucose and hypoxia/reoxygenation (H/R) groups compared with that in the control or high glucose-treated groups. Pemafibrate treatment significantly enhanced the ATP and superoxide dismutase levels, and reduced the mitochondrial reactive oxygen species and malondialdehyde levels compared with the high glucose combined with H/R group. Furthermore, pemafibrate inhibited the expression of cytochrome c and cleaved-caspase-3, indicating its involvement in the regulation of mitochondrial apoptosis. Pemafibrate also reduced the expression of nuclear factor-κB (NF-κB), the activation of which reversed the protective effects of pemafibrate on diabetic myocardial IR injury in vitro. Taken together, these results suggested that pemafibrate may activate PPARα to protect the T1DM rat myocardium against IR injury through inhibition of NF-κB signaling.
Keywords: apoptosis; diabetes; myocardial ischemia-reperfusion injury; oxidative stress; peroxisome proliferator-activated receptor α.
Copyright: © Li et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Peroxisome proliferator-activated receptor γ (PPARγ) mediates the protective effect of quercetin against myocardial ischemia-reperfusion injury via suppressing the NF-κB pathway.Am J Transl Res. 2016 Dec 15;8(12):5169-5186. eCollection 2016. Am J Transl Res. 2016. PMID: 28077993 Free PMC article.
-
Aucubin Attenuates Liver Ischemia-Reperfusion Injury by Inhibiting the HMGB1/TLR-4/NF-κB Signaling Pathway, Oxidative Stress, and Apoptosis.Front Pharmacol. 2020 Sep 8;11:544124. doi: 10.3389/fphar.2020.544124. eCollection 2020. Front Pharmacol. 2020. PMID: 33013386 Free PMC article.
-
AMPK Contributes to Cardioprotective Effects of Pterostilbene Against Myocardial Ischemia- Reperfusion Injury in Diabetic Rats by Suppressing Cardiac Oxidative Stress and Apoptosis.Cell Physiol Biochem. 2018;46(4):1381-1397. doi: 10.1159/000489154. Epub 2018 Apr 18. Cell Physiol Biochem. 2018. PMID: 29689567
-
(-)-Epigallocatechin-3-gallate attenuates myocardial injury induced by ischemia/reperfusion in diabetic rats and in H9c2 cells under hyperglycemic conditions.Int J Mol Med. 2017 Aug;40(2):389-399. doi: 10.3892/ijmm.2017.3014. Epub 2017 Jun 8. Int J Mol Med. 2017. PMID: 28714516 Free PMC article.
-
PPARγ in Ischemia-Reperfusion Injury: Overview of the Biology and Therapy.Front Pharmacol. 2021 Apr 28;12:600618. doi: 10.3389/fphar.2021.600618. eCollection 2021. Front Pharmacol. 2021. PMID: 33995008 Free PMC article. Review.
Cited by
-
Cardioprotective effects of sinomenine in myocardial ischemia/reperfusion injury in a rat model.Saudi Pharm J. 2022 Jun;30(6):669-678. doi: 10.1016/j.jsps.2022.04.005. Epub 2022 Apr 21. Saudi Pharm J. 2022. PMID: 35812144 Free PMC article.
References
-
- Bhawal UK, Yoshida K, Kurita T, Suzuki M, Okada Y, Tewari N, Oka S, Kuboyama N, Hiratsuka K. Effects of 830 nm low-power laser irradiation on body weight gain and inflammatory cytokines in experimental diabetes in different animal models. Laser Ther. 2019;28:257–265. doi: 10.5978/islsm.19-OR-17. - DOI - PMC - PubMed
-
- Šimonienė D, Platūkiene A, Prakapienė E, Radzevičienė L, Veličkiene D. Insulin resistance in type 1 diabetes mellitus and its association with patient's micro- and macrovascular complications, sex hormones, and other clinical data. Diabetes Ther. 2020;11:161–174. doi: 10.1007/s13300-019-00729-5. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
