Heat Shock Tolerance in Deschampsia antarctica Desv. Cultivated in vitro Is Mediated by Enzymatic and Non-enzymatic Antioxidants
- PMID: 33732277
- PMCID: PMC7959801
- DOI: 10.3389/fpls.2021.635491
Heat Shock Tolerance in Deschampsia antarctica Desv. Cultivated in vitro Is Mediated by Enzymatic and Non-enzymatic Antioxidants
Abstract
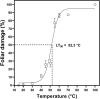
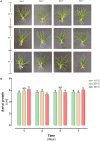
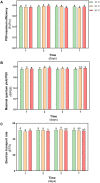
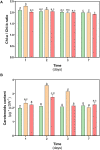
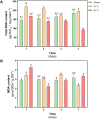
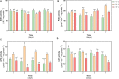
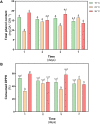
Deschampsia antarctica Desv, is the most successful colonizing species of a cold continent. In recent years due to climate change, the frequency of heat waves has increased in Antarctica, registering anomalous high temperatures during the summer of 2020. However, the populations of D. antarctica are responding positively to these events, increasing in number and size throughout the Antarctic Peninsula. In this work, the physiological and biochemical responses of D. antarctica plants grown in vitro (15 ± 1°C) and plants subjected to two heat shock treatments (23 and 35°C) were evaluated. The results obtained show that D. antarctica grown in vitro is capable of tolerating heat shock treatments; without showing visible damage to its morphology, or changes in its oxidative state and photosynthetic performance. These tolerance responses are primarily mediated by the efficient role of enzymatic and non-enzymatic antioxidant systems that maintain redox balance at higher temperatures. It is postulated that these mechanisms also operate in plants under natural conditions when exposed to environmental stresses.
Keywords: Antarctica; climate change; oxidative stress–related enzymes; peroxidases; photosynthesis.
Copyright © 2021 Cortés-Antiquera, Pizarro, Contreras, Köhler and Zúñiga.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Antioxidant Responses Induced by UVB Radiation in Deschampsia antarctica Desv.Front Plant Sci. 2017 May 31;8:921. doi: 10.3389/fpls.2017.00921. eCollection 2017. Front Plant Sci. 2017. PMID: 28620407 Free PMC article.
-
Effects of temperature and water availability on light energy utilization in photosynthetic processes of Deschampsia antarctica.Physiol Plant. 2019 Mar;165(3):511-523. doi: 10.1111/ppl.12739. Epub 2018 Jul 19. Physiol Plant. 2019. PMID: 29602170
-
The role of photochemical quenching and antioxidants in photoprotection of Deschampsia antarctica.Funct Plant Biol. 2004 Aug;31(7):731-741. doi: 10.1071/FP03082. Funct Plant Biol. 2004. PMID: 32688943
-
Ecophysiology of Antarctic Vascular Plants: An Update on the Extreme Environment Resistance Mechanisms and Their Importance in Facing Climate Change.Plants (Basel). 2024 Feb 3;13(3):449. doi: 10.3390/plants13030449. Plants (Basel). 2024. PMID: 38337983 Free PMC article. Review.
-
Trace metals in Antarctica related to climate change and increasing human impact.Rev Environ Contam Toxicol. 2000;166:129-73. Rev Environ Contam Toxicol. 2000. PMID: 10868078 Review.
Cited by
-
Interactive effects of changes in UV radiation and climate on terrestrial ecosystems, biogeochemical cycles, and feedbacks to the climate system.Photochem Photobiol Sci. 2023 May;22(5):1049-1091. doi: 10.1007/s43630-023-00376-7. Epub 2023 Feb 1. Photochem Photobiol Sci. 2023. PMID: 36723799 Free PMC article.
References
-
- Asami D., Hong Y., Barrett D., Mitchell A. (2003). Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried conventional, organic, and sustainable agricultural practices. J. Agricult. Food Chem. 51 1237–1241. - PubMed
-
- Beauchamp C., Fridovich I. (1971). Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44 276–287. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

