S100A4 in Spinal Substantia Gelatinosa from Dorsal Root Ganglia Modulates Neuropathic Pain in a Rodent Spinal Nerve Injury Model
- PMID: 33732013
- PMCID: PMC7956897
- DOI: 10.2147/JPR.S293462
S100A4 in Spinal Substantia Gelatinosa from Dorsal Root Ganglia Modulates Neuropathic Pain in a Rodent Spinal Nerve Injury Model
Abstract
Purpose: To detect the spatio-temporal expression of S100A4 in a spinal nerve ligation (SNL) rat model. Also to figure out which other molecules directly interact with S100A4 to explore the possible mechanisms which might be involved in neuropathic pain.
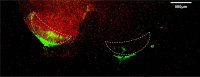
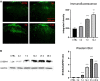
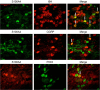
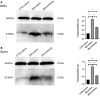
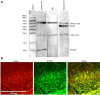
Methods: Seven-week-old male SD rats were used for the SNL model construction. Immunofluorescence and Western blotting were used to detect the spatio-temporal expression of S100A4 in the model. S100A4 was co-labeled with a number of related molecules and marker molecules that can distinguish between cell types. After intrathecal injection of S100A4 neutralizing antibody, the behavioral changes of SNL rats were recorded, and molecular changes compared. The direct interaction between S100A4 and other related molecules was verified by co-immunoprecipitation (co-IP) to explore its possible mechanism.
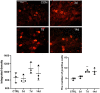
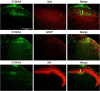
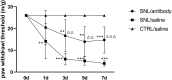
Results: After spinal nerve ligation, the content of S100A4 in the dorsal root ganglion (DRG) and spinal dorsal horn increased significantly. Intrathecal injection of S100A4 neutralizing antibody could effectively relieve the mechanical pain in rats. co-IP revealed a direct interaction between S100A4 and RAGE.
Conclusion: The content of S100A4 in the DRG and spinal dorsal horn of SNL rats increased, compared with that of the control group. Intrathecal injection of S100A4 neutralizing antibody could effectively relieve the mechanical pain in SNL rats. S100A4 may be involved in the production of neuropathic pain through RAGE or other ways, but the specific mechanism needs to be further studied.
Keywords: S100 calcium binding protein A4; neuropathic pain; receptor for advanced glycation end products; spinal nerve ligation model.
© 2021 Jiang et al.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to this work, the research, authorship, or publication of this article.
Figures
Similar articles
-
Enhanced RAGE Expression in the Dorsal Root Ganglion May Contribute to Neuropathic Pain Induced by Spinal Nerve Ligation in Rats.Pain Med. 2016 May;17(5):803-12. doi: 10.1093/pm/pnv035. Epub 2015 Dec 7. Pain Med. 2016. PMID: 26814270
-
PARP-1-regulated TNF-α expression in the dorsal root ganglia and spinal dorsal horn contributes to the pathogenesis of neuropathic pain in rats.Brain Behav Immun. 2020 Aug;88:482-496. doi: 10.1016/j.bbi.2020.04.019. Epub 2020 Apr 10. Brain Behav Immun. 2020. PMID: 32283287
-
MicroRNA-124-3p attenuates the development of nerve injury-induced neuropathic pain by targeting early growth response 1 in the dorsal root ganglia and spinal dorsal horn.J Neurochem. 2021 Aug;158(4):928-942. doi: 10.1111/jnc.15433. Epub 2021 Jun 20. J Neurochem. 2021. PMID: 34008206
-
CXCL12/CXCR4 signaling contributes to neuropathic pain via central sensitization mechanisms in a rat spinal nerve ligation model.CNS Neurosci Ther. 2019 Sep;25(9):922-936. doi: 10.1111/cns.13128. Epub 2019 Apr 7. CNS Neurosci Ther. 2019. PMID: 30955244 Free PMC article.
-
Increased Expression of Fibronectin Leucine-Rich Transmembrane Protein 3 in the Dorsal Root Ganglion Induces Neuropathic Pain in Rats.J Neurosci. 2019 Sep 18;39(38):7615-7627. doi: 10.1523/JNEUROSCI.0295-19.2019. Epub 2019 Jul 25. J Neurosci. 2019. PMID: 31346030 Free PMC article.
Cited by
-
BRD4 Inhibition Attenuates Inflammatory Pain by Ameliorating NLRP3 Inflammasome-Induced Pyroptosis.Front Immunol. 2022 Jan 26;13:837977. doi: 10.3389/fimmu.2022.837977. eCollection 2022. Front Immunol. 2022. PMID: 35154163 Free PMC article.
-
Huc-MSCs-derived exosomes attenuate inflammatory pain by regulating microglia pyroptosis and autophagy via the miR-146a-5p/TRAF6 axis.J Nanobiotechnology. 2022 Jul 14;20(1):324. doi: 10.1186/s12951-022-01522-6. J Nanobiotechnology. 2022. PMID: 35836229 Free PMC article.
-
Neuropeptide Y in the amygdala contributes to neuropathic pain-like behaviors in rats via the neuropeptide Y receptor type 2/mitogen-activated protein kinase axis.Bioengineered. 2022 Apr;13(4):8101-8114. doi: 10.1080/21655979.2022.2051783. Bioengineered. 2022. PMID: 35313782 Free PMC article.
-
S100 proteins: a new frontier in fibromyalgia research.Mol Brain. 2024 May 26;17(1):29. doi: 10.1186/s13041-024-01102-9. Mol Brain. 2024. PMID: 38797848 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

