Puerarin Attenuates LPS-Induced Inflammatory Responses and Oxidative Stress Injury in Human Umbilical Vein Endothelial Cells through Mitochondrial Quality Control
- PMID: 33728025
- PMCID: PMC7937474
- DOI: 10.1155/2021/6659240
Puerarin Attenuates LPS-Induced Inflammatory Responses and Oxidative Stress Injury in Human Umbilical Vein Endothelial Cells through Mitochondrial Quality Control
Retraction in
-
Retracted: Puerarin Attenuates LPS-Induced Inflammatory Responses and Oxidative Stress Injury in Human Umbilical Vein Endothelial Cells through Mitochondrial Quality Control.Oxid Med Cell Longev. 2023 Oct 11;2023:9767123. doi: 10.1155/2023/9767123. eCollection 2023. Oxid Med Cell Longev. 2023. PMID: 37868737 Free PMC article.
Abstract
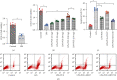
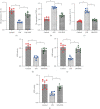
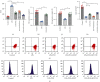
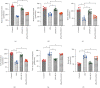
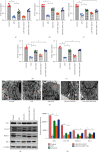
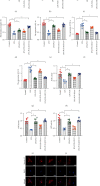
Atherosclerosis is closely associated with the inflammatory reaction of vascular endothelial cells. Puerarin (Pue), the main active component isolated from the rhizome of Pueraria lobata, is an isoflavone compound with potent antioxidant properties. Although Pue exhibits promising antiatherosclerotic pharmacological effects, only a few studies have reported its protective effect on endothelial cells. This study found that Pue could partly regulate mitochondrial function in human umbilical vein endothelial cells (HUVECs) and reduce or inhibit lipopolysaccharide-induced inflammatory reactions and oxidative stress injury in HUVECs, likely via mitochondrial quality control. Furthermore, the protective effect of Pue on HUVECs was closely related to the SIRT-1 signaling pathway. Pue increased autophagy and mitochondrial antioxidant potential via increased SIRT-1 expression, reducing excessive production of ROS and inhibiting the expression of inflammatory factors and oxidative stress injury. Therefore, Pue may improve mitochondrial respiratory function and energy metabolism, increasing the vulnerability of HUVECs to an inflammatory state.
Copyright © 2021 Xing Chang et al.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
An isoflavonoid-enriched extract from Pueraria lobata (kudzu) root protects human umbilical vein endothelial cells against oxidative stress induced apoptosis.J Ethnopharmacol. 2016 Dec 4;193:524-530. doi: 10.1016/j.jep.2016.10.005. Epub 2016 Oct 4. J Ethnopharmacol. 2016. PMID: 27717903
-
Puerarin mitigated LPS-ATP or HG-primed endothelial cells damage and diabetes-associated cardiovascular disease via ROS-NLRP3 signalling.J Cell Mol Med. 2024 May;28(10):e18239. doi: 10.1111/jcmm.18239. J Cell Mol Med. 2024. PMID: 38774996 Free PMC article.
-
Allicin Decreases Lipopolysaccharide-Induced Oxidative Stress and Inflammation in Human Umbilical Vein Endothelial Cells through Suppression of Mitochondrial Dysfunction and Activation of Nrf2.Cell Physiol Biochem. 2017;41(6):2255-2267. doi: 10.1159/000475640. Epub 2017 Apr 26. Cell Physiol Biochem. 2017. PMID: 28456799
-
Puerarin alleviates acrolein-induced atherosclerosis by activating the MYH9-mediated SIRT1/Nrf2 cascade to inhibit the activation of inflammasome.Biotechnol Appl Biochem. 2024 Oct;71(5):1129-1138. doi: 10.1002/bab.2603. Epub 2024 May 23. Biotechnol Appl Biochem. 2024. PMID: 38783542
-
Relevance of real-time analyzers to determine mitochondrial quality in endothelial cells and oxidative stress in preeclampsia.Vascul Pharmacol. 2024 Jun;155:107372. doi: 10.1016/j.vph.2024.107372. Epub 2024 Apr 6. Vascul Pharmacol. 2024. PMID: 38583694 Review.
Cited by
-
B-cell lymphoma-2 phosphorylation at Ser70 site-related autophagy mediates puerarin-inhibited the apoptosis of MC3T3-E1 cells during osteoblastogenesis.J Tradit Chin Med. 2024 Feb;44(1):27-34. doi: 10.19852/j.cnki.jtcm.20231024.002. J Tradit Chin Med. 2024. PMID: 38213236 Free PMC article.
-
Puerarin inhibits Staphylococcus aureus-induced endometritis through attenuating inflammation and ferroptosis via regulating the P2X7/NLRP3 signalling pathway.J Cell Mol Med. 2024 Jul;28(14):e18550. doi: 10.1111/jcmm.18550. J Cell Mol Med. 2024. PMID: 39042561 Free PMC article.
-
Puerarin Alleviates UUO-Induced Inflammation and Fibrosis by Regulating the NF-κB P65/STAT3 and TGFβ1/Smads Signaling Pathways.Drug Des Devel Ther. 2021 Aug 24;15:3697-3708. doi: 10.2147/DDDT.S321879. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 34465981 Free PMC article.
-
Puerarin alleviates apoptosis and inflammation in kidney stone cells via the PI3K/AKT pathway: Network pharmacology and experimental verification.J Cell Mol Med. 2024 Oct;28(20):e70180. doi: 10.1111/jcmm.70180. J Cell Mol Med. 2024. PMID: 39462270 Free PMC article.
-
Trained immunity in monocyte/macrophage: Novel mechanism of phytochemicals in the treatment of atherosclerotic cardiovascular disease.Front Pharmacol. 2023 Feb 21;14:1109576. doi: 10.3389/fphar.2023.1109576. eCollection 2023. Front Pharmacol. 2023. PMID: 36895942 Free PMC article. Review.
References
-
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. - PubMed
-
- Zhu H., Li Y., Wang M. X., Wang J. H., Du W. X., Zhou F. Analysis of cardiovascular disease-related NF-κB-regulated genes and microRNAs in TNFα-treated primary mouse vascular endothelial cells. Journal of Zhejiang University-Science B. 2019;20(10):803–815. doi: 10.1631/jzus.b1800631. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

