Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant
- PMID: 33725432
- PMCID: PMC7993410
- DOI: 10.1056/NEJMoa2102214
Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant
Abstract
Background: Assessment of the safety and efficacy of vaccines against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in different populations is essential, as is investigation of the efficacy of the vaccines against emerging SARS-CoV-2 variants of concern, including the B.1.351 (501Y.V2) variant first identified in South Africa.
Methods: We conducted a multicenter, double-blind, randomized, controlled trial to assess the safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) in people not infected with the human immunodeficiency virus (HIV) in South Africa. Participants 18 to less than 65 years of age were assigned in a 1:1 ratio to receive two doses of vaccine containing 5×1010 viral particles or placebo (0.9% sodium chloride solution) 21 to 35 days apart. Serum samples obtained from 25 participants after the second dose were tested by pseudovirus and live-virus neutralization assays against the original D614G virus and the B.1.351 variant. The primary end points were safety and efficacy of the vaccine against laboratory-confirmed symptomatic coronavirus 2019 illness (Covid-19) more than 14 days after the second dose.
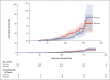
Results: Between June 24 and November 9, 2020, we enrolled 2026 HIV-negative adults (median age, 30 years); 1010 and 1011 participants received at least one dose of placebo or vaccine, respectively. Both the pseudovirus and the live-virus neutralization assays showed greater resistance to the B.1.351 variant in serum samples obtained from vaccine recipients than in samples from placebo recipients. In the primary end-point analysis, mild-to-moderate Covid-19 developed in 23 of 717 placebo recipients (3.2%) and in 19 of 750 vaccine recipients (2.5%), for an efficacy of 21.9% (95% confidence interval [CI], -49.9 to 59.8). Among the 42 participants with Covid-19, 39 cases (95.1% of 41 with sequencing data) were caused by the B.1.351 variant; vaccine efficacy against this variant, analyzed as a secondary end point, was 10.4% (95% CI, -76.8 to 54.8). The incidence of serious adverse events was balanced between the vaccine and placebo groups.
Conclusions: A two-dose regimen of the ChAdOx1 nCoV-19 vaccine did not show protection against mild-to-moderate Covid-19 due to the B.1.351 variant. (Funded by the Bill and Melinda Gates Foundation and others; ClinicalTrials.gov number, NCT04444674; Pan African Clinical Trials Registry number, PACTR202006922165132).
Copyright © 2021 Massachusetts Medical Society.
Figures

Comment in
-
In South Africa, a 2-dose Oxford/AZ vaccine did not prevent mild to moderate COVID-19 (cases mainly B.1.351 variant).Ann Intern Med. 2021 May;174(5):JC50. doi: 10.7326/ACPJ202105180-050. Epub 2021 May 4. Ann Intern Med. 2021. PMID: 33939483
-
Interplay between Emerging SARS-CoV-2 Variants and Pandemic Control.N Engl J Med. 2021 May 20;384(20):1952-1954. doi: 10.1056/NEJMe2103931. Epub 2021 May 5. N Engl J Med. 2021. PMID: 33951359 No abstract available.
-
ChAdOx1 nCoV-19 Vaccine Efficacy against the B.1.351 Variant.N Engl J Med. 2021 Aug 5;385(6):571. doi: 10.1056/NEJMc2110093. Epub 2021 Jul 21. N Engl J Med. 2021. PMID: 34289270 No abstract available.
Similar articles
-
Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: an interim analysis of a randomised, double-blind, placebo-controlled, phase 1B/2A trial.Lancet HIV. 2021 Sep;8(9):e568-e580. doi: 10.1016/S2352-3018(21)00157-0. Epub 2021 Aug 17. Lancet HIV. 2021. PMID: 34416193 Free PMC article. Clinical Trial.
-
Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials.Lancet. 2021 Mar 6;397(10277):881-891. doi: 10.1016/S0140-6736(21)00432-3. Epub 2021 Feb 19. Lancet. 2021. PMID: 33617777 Free PMC article.
-
Safety and efficacy of the intranasal spray SARS-CoV-2 vaccine dNS1-RBD: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial.Lancet Respir Med. 2023 Dec;11(12):1075-1088. doi: 10.1016/S2213-2600(23)00349-1. Epub 2023 Nov 15. Lancet Respir Med. 2023. PMID: 37979588 Free PMC article. Clinical Trial.
-
Infliximab for maintenance of medically-induced remission in Crohn's disease.Cochrane Database Syst Rev. 2024 Feb 19;2(2):CD012609. doi: 10.1002/14651858.CD012609.pub2. Cochrane Database Syst Rev. 2024. PMID: 38372447 Review.
-
Pharmacological treatments in panic disorder in adults: a network meta-analysis.Cochrane Database Syst Rev. 2023 Nov 28;11(11):CD012729. doi: 10.1002/14651858.CD012729.pub3. Cochrane Database Syst Rev. 2023. PMID: 38014714 Free PMC article. Review.
Cited by
-
Humoral and Cellular Immune Response after Three Doses of Sinopharm [Vero Cell]-Inactivated COVID-19 Vaccine in Combination with SARS-CoV-2 Infection Leads to Hybrid Immunity.Pharmaceuticals (Basel). 2024 Jan 17;17(1):122. doi: 10.3390/ph17010122. Pharmaceuticals (Basel). 2024. PMID: 38256955 Free PMC article.
-
SARS-CoV-2: pathogenesis, therapeutics, variants, and vaccines.Front Microbiol. 2024 Jun 13;15:1334152. doi: 10.3389/fmicb.2024.1334152. eCollection 2024. Front Microbiol. 2024. PMID: 38939189 Free PMC article. Review.
-
Cytokine Storm: The Primary Determinant for the Pathophysiological Evolution of COVID-19 Deterioration.Front Immunol. 2021 Apr 28;12:589095. doi: 10.3389/fimmu.2021.589095. eCollection 2021. Front Immunol. 2021. PMID: 33995341 Free PMC article. Review.
-
Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern in real-world: a literature review and meta-analysis.Emerg Microbes Infect. 2022 Dec;11(1):2383-2392. doi: 10.1080/22221751.2022.2122582. Emerg Microbes Infect. 2022. PMID: 36069511 Free PMC article.
-
SARS-CoV-2 501Y.V2 (B.1.351) elicits cross-reactive neutralizing antibodies.bioRxiv [Preprint]. 2021 Mar 11:2021.03.06.434193. doi: 10.1101/2021.03.06.434193. bioRxiv. 2021. Update in: N Engl J Med. 2021 Jun 3;384(22):2161-2163. doi: 10.1056/NEJMc2104192 PMID: 33688657 Free PMC article. Updated. Preprint.
References
-
- Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 vaccines at pandemic speed. N Engl J Med 2020;382:1969-1973. - PubMed
-
- Collins FS, Stoffels P. Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV): an unprecedented partnership for unprecedented times. JAMA 2020;323:2455-2457. - PubMed
-
- Lurie N, Sharfstein JM, Goodman JL. The development of COVID-19 vaccines: safeguards needed. JAMA 2020. July 6 (Epub ahead of print). - PubMed
-
- Pfizer. Pfizer and BioNTech announce vaccine candidate against COVID-19 achieved success in first interim analysis from phase 3 study. November 9, 2020. (https://www.pfizer.com/news/press-release/press-release-detail/pfizer-an...).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
