Underlying mechanisms and drug intervention strategies for the tumour microenvironment
- PMID: 33722297
- PMCID: PMC7962349
- DOI: 10.1186/s13046-021-01893-y
Underlying mechanisms and drug intervention strategies for the tumour microenvironment
Abstract
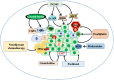
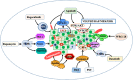
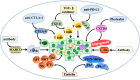
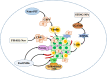
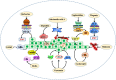
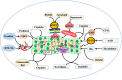
Cancer occurs in a complex tissue environment, and its progression depends largely on the tumour microenvironment (TME). The TME has a highly complex and comprehensive system accompanied by dynamic changes and special biological characteristics, such as hypoxia, nutrient deficiency, inflammation, immunosuppression and cytokine production. In addition, a large number of cancer-associated biomolecules and signalling pathways are involved in the above bioprocesses. This paper reviews our understanding of the TME and describes its biological and molecular characterization in different stages of cancer development. Furthermore, we discuss in detail the intervention strategies for the critical points of the TME, including chemotherapy, targeted therapy, immunotherapy, natural products from traditional Chinese medicine, combined drug therapy, etc., providing a scientific basis for cancer therapy from the perspective of key molecular targets in the TME.
Keywords: Cancer development; Drug intervention strategies; Molecular targets; TME.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
The role of macrophage in regulating tumour microenvironment and the strategies for reprogramming tumour-associated macrophages in antitumour therapy.Eur J Cell Biol. 2021 Mar;100(2):151153. doi: 10.1016/j.ejcb.2021.151153. Epub 2021 Jan 13. Eur J Cell Biol. 2021. PMID: 33476912 Review.
-
Taking a Full Snapshot of Cancer Biology: Deciphering the Tumor Microenvironment for Effective Cancer Therapy in the Oncology Clinic.OMICS. 2020 Apr;24(4):175-179. doi: 10.1089/omi.2020.0019. Epub 2020 Mar 13. OMICS. 2020. PMID: 32176591 Review.
-
Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy.Ann Oncol. 2016 Aug;27(8):1482-92. doi: 10.1093/annonc/mdw168. Epub 2016 Apr 10. Ann Oncol. 2016. PMID: 27069014 Review.
-
Targeting cancer-related inflammation in the era of immunotherapy.Immunol Cell Biol. 2017 Apr;95(4):325-332. doi: 10.1038/icb.2016.126. Epub 2017 Jan 10. Immunol Cell Biol. 2017. PMID: 27999432 Review.
-
The Roles of CD38 and CD157 in the Solid Tumor Microenvironment and Cancer Immunotherapy.Cells. 2019 Dec 20;9(1):26. doi: 10.3390/cells9010026. Cells. 2019. PMID: 31861847 Free PMC article. Review.
Cited by
-
The G Protein Estrogen Receptor (GPER) is involved in the resistance to the CDK4/6 inhibitor palbociclib in breast cancer.J Exp Clin Cancer Res. 2024 Jun 18;43(1):171. doi: 10.1186/s13046-024-03096-7. J Exp Clin Cancer Res. 2024. PMID: 38886784 Free PMC article.
-
Inflammation and tumor progression: signaling pathways and targeted intervention.Signal Transduct Target Ther. 2021 Jul 12;6(1):263. doi: 10.1038/s41392-021-00658-5. Signal Transduct Target Ther. 2021. PMID: 34248142 Free PMC article.
-
Unveiling the role of osteosarcoma-derived secretome in premetastatic lung remodelling.J Exp Clin Cancer Res. 2023 Nov 30;42(1):328. doi: 10.1186/s13046-023-02886-9. J Exp Clin Cancer Res. 2023. PMID: 38031171 Free PMC article.
-
CD8/PD-L1 immunohistochemical reactivity and gene alterations in cutaneous squamous cell carcinoma.PLoS One. 2023 Feb 13;18(2):e0281647. doi: 10.1371/journal.pone.0281647. eCollection 2023. PLoS One. 2023. PMID: 36780540 Free PMC article.
-
Roles of the CXCL8-CXCR1/2 Axis in the Tumor Microenvironment and Immunotherapy.Molecules. 2021 Dec 27;27(1):137. doi: 10.3390/molecules27010137. Molecules. 2021. PMID: 35011369 Free PMC article. Review.
References
-
- Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8(2):98–101. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

