The time to offer treatments for COVID-19
- PMID: 33721548
- PMCID: PMC8074648
- DOI: 10.1080/13543784.2021.1901883
The time to offer treatments for COVID-19
Abstract
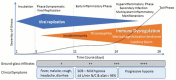
Background: COVID-19 has several overlapping phases. Treatments to date have focused on the late stage of disease in hospital. Yet, the pandemic is by propagated by the viral phase in out-patients. The current public health strategy relies solely on vaccines to prevent disease.Methods: We searched the major national registries, pubmed.org, and the preprint servers for all ongoing, completed and published trial results.Results: As of 2/15/2021, we found 111 publications reporting findings on 14 classes of agents, and 9 vaccines. There were 62 randomized controlled studies, the rest retrospective observational analyses. Only 21 publications dealt with outpatient care. Remdesivir and high titer convalescent plasma have emergency use authorization for hospitalized patients in the U.S.A. There is also support for glucocorticoid treatment of the COVID-19 respiratory distress syndrome. Monoclonal antibodies are authorized for outpatients, but supply is inadequate to treat all at time of diagnosis. Favipiravir, ivermectin, and interferons are approved in certain countries.Expert Opinion: Vaccines and antibodies are highly antigen specific, and new SARS-Cov-2 variants are appearing. We call on public health authorities to authorize treatments with known low-risk and possible benefit for outpatients in parallel with universal vaccination.
Keywords: SARS-Cov-2; convalescent plasma; covid-19; favipiravir; hcq; interferon-β-1; interferon-λ; ivermectin; remdesivir; synthetic anti-spike protein antibodies.
Figures

Similar articles
-
Reconvalescent plasma/camostat mesylate in early SARS-CoV-2 Q-PCR positive high-risk individuals (RES-Q-HR): a structured summary of a study protocol for a randomized controlled trial.Trials. 2021 May 17;22(1):343. doi: 10.1186/s13063-021-05181-0. Trials. 2021. PMID: 34001215 Free PMC article.
-
Evaluating the efficacy and safety of human anti-SARS-CoV-2 convalescent plasma in severely ill adults with COVID-19: A structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Jun 8;21(1):499. doi: 10.1186/s13063-020-04422-y. Trials. 2020. PMID: 32513308 Free PMC article.
-
Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7.Nature. 2021 May;593(7857):130-135. doi: 10.1038/s41586-021-03398-2. Epub 2021 Mar 8. Nature. 2021. PMID: 33684923
-
SARS-CoV-2: Immune Response Elicited by Infection and Development of Vaccines and Treatments.Front Immunol. 2020 Dec 11;11:569760. doi: 10.3389/fimmu.2020.569760. eCollection 2020. Front Immunol. 2020. PMID: 33362758 Free PMC article. Review.
-
An update of anti-viral treatment of COVID-19.Turk J Med Sci. 2021 Dec 17;51(SI-1):3372-3390. doi: 10.3906/sag-2106-250. Turk J Med Sci. 2021. PMID: 34391321 Free PMC article. Review.
Cited by
-
Advances in attractive therapeutic approach for macrophage activation syndrome in COVID-19.Front Immunol. 2023 Jul 6;14:1200289. doi: 10.3389/fimmu.2023.1200289. eCollection 2023. Front Immunol. 2023. PMID: 37483597 Free PMC article. Review.
-
Post-COVID-19 Anosmia and Therapies: Stay Tuned for New Drugs to Sniff Out.Diseases. 2023 May 27;11(2):79. doi: 10.3390/diseases11020079. Diseases. 2023. PMID: 37366867 Free PMC article. Review.
-
Bacterial infections in patients hospitalized with COVID-19.Intern Emerg Med. 2022 Mar;17(2):431-438. doi: 10.1007/s11739-021-02824-7. Epub 2021 Aug 18. Intern Emerg Med. 2022. PMID: 34406633 Free PMC article.
-
The Value of Ursodeoxycholic Acid and Mesenchymal Stem Cells in the Treatment of Severe COVID-19.Microorganisms. 2024 Jun 22;12(7):1269. doi: 10.3390/microorganisms12071269. Microorganisms. 2024. PMID: 39065038 Free PMC article.
-
Combination therapies for COVID-19: An overview of the clinical trials landscape.Br J Clin Pharmacol. 2022 Feb;88(4):1590-1597. doi: 10.1111/bcp.15089. Epub 2021 Oct 17. Br J Clin Pharmacol. 2022. PMID: 34558094 Free PMC article. Review.
References
-
- WHO Coronavirus Disease (COVID-19) Dashboard 2021. Data last updated: 2021 February 15, 11 pm, Greenwich mean time
-
- Griffin DO, Brennan-Rieder D, Ngo B, et al. The importance of understanding the stages of COVID-19 in treatment and trials. AIDS Rev. 2021. DOI:10.24875/AIDSRev.200001261. - DOI - PubMed
-
•• This article presents COVID-19 as a disease with specific phases and calls for research on distinctive treatments of different phases rather than to be viewed as a single process.
-
- Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
