Endothelial Cells in Emerging Viral Infections
- PMID: 33718448
- PMCID: PMC7943456
- DOI: 10.3389/fcvm.2021.619690
Endothelial Cells in Emerging Viral Infections
Abstract
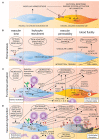
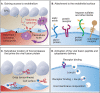
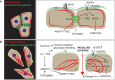
There are several reasons to consider the role of endothelial cells in COVID-19 and other emerging viral infections. First, severe cases of COVID-19 show a common breakdown of central vascular functions. Second, SARS-CoV-2 replicates in endothelial cells. Third, prior deterioration of vascular function exacerbates disease, as the most common comorbidities of COVID-19 (obesity, hypertension, and diabetes) are all associated with endothelial dysfunction. Importantly, SARS-CoV-2's ability to infect endothelium is shared by many emerging viruses, including henipaviruses, hantavirus, and highly pathogenic avian influenza virus, all specifically targeting endothelial cells. The ability to infect endothelium appears to support generalised dissemination of infection and facilitate the access to certain tissues. The disturbed vascular function observed in severe COVID-19 is also a prominent feature of many other life-threatening viral diseases, underscoring the need to understand how viruses modulate endothelial function. We here review the role of vascular endothelial cells in emerging viral infections, starting with a summary of endothelial cells as key mediators and regulators of vascular and immune responses in health and infection. Next, we discuss endotheliotropism as a possible virulence factor and detail features that regulate viruses' ability to attach to and enter endothelial cells. We move on to review how endothelial cells detect invading viruses and respond to infection, with particular focus on pathways that may influence vascular function and the host immune system. Finally, we discuss how endothelial cell function can be dysregulated in viral disease, either by viral components or as bystander victims of overshooting or detrimental inflammatory and immune responses. Many aspects of how viruses interact with the endothelium remain poorly understood. Considering the diversity of such mechanisms among different emerging viruses allows us to highlight common features that may be of general validity and point out important challenges.
Keywords: SARS-CoV-2; emerging infections; endothelium; inflammation; vascular dysfunction and damage; virus.
Copyright © 2021 Fosse, Haraldsen, Falk and Edelmann.
Conflict of interest statement
KF was employed by the company AquaMed Consulting AS. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Recombinant ACE2 Expression Is Required for SARS-CoV-2 To Infect Primary Human Endothelial Cells and Induce Inflammatory and Procoagulative Responses.mBio. 2020 Dec 11;11(6):e03185-20. doi: 10.1128/mBio.03185-20. mBio. 2020. PMID: 33310781 Free PMC article.
-
Endothelial Dysfunction as a Primary Consequence of SARS-CoV-2 Infection.Adv Exp Med Biol. 2021;1321:33-43. doi: 10.1007/978-3-030-59261-5_3. Adv Exp Med Biol. 2021. PMID: 33656711
-
Assessing the application of a pseudovirus system for emerging SARS-CoV-2 and re-emerging avian influenza virus H5 subtypes in vaccine development.Biomed J. 2020 Aug;43(4):375-387. doi: 10.1016/j.bj.2020.06.003. Epub 2020 Jun 6. Biomed J. 2020. PMID: 32611537 Free PMC article.
-
Vitamin D deficiency in association with endothelial dysfunction: Implications for patients with COVID-19.Rev Cardiovasc Med. 2020 Sep 30;21(3):339-344. doi: 10.31083/j.rcm.2020.03.131. Rev Cardiovasc Med. 2020. PMID: 33070539 Review.
-
Interactions of Influenza and SARS-CoV-2 with the Lung Endothelium: Similarities, Differences, and Implications for Therapy.Viruses. 2021 Jan 22;13(2):161. doi: 10.3390/v13020161. Viruses. 2021. PMID: 33499234 Free PMC article. Review.
Cited by
-
Restoration of vascular endothelial integrity by mesenchymal stromal/stem cells in debilitating virus diseases.Hum Cell. 2022 Nov;35(6):1633-1639. doi: 10.1007/s13577-022-00785-3. Epub 2022 Sep 6. Hum Cell. 2022. PMID: 36068397 Free PMC article. Review.
-
Viral infections in etiology of mental disorders: a broad analysis of cytokine profile similarities - a narrative review.Front Cell Infect Microbiol. 2024 Aug 14;14:1423739. doi: 10.3389/fcimb.2024.1423739. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39206043 Free PMC article. Review.
-
Interferon-alpha or -beta facilitates SARS-CoV-2 pulmonary vascular infection by inducing ACE2.Angiogenesis. 2022 May;25(2):225-240. doi: 10.1007/s10456-021-09823-4. Epub 2021 Oct 29. Angiogenesis. 2022. PMID: 34714440 Free PMC article.
-
Porcine Epidemic Diarrhea Virus Infection Disrupts the Nasal Endothelial Barrier To Favor Viral Dissemination.J Virol. 2022 May 11;96(9):e0038022. doi: 10.1128/jvi.00380-22. Epub 2022 Apr 18. J Virol. 2022. PMID: 35435723 Free PMC article.
-
Serratiopeptidase Attenuates Lipopolysaccharide-Induced Vascular Inflammation by Inhibiting the Expression of Monocyte Chemoattractant Protein-1.Curr Issues Mol Biol. 2023 Mar 8;45(3):2201-2212. doi: 10.3390/cimb45030142. Curr Issues Mol Biol. 2023. PMID: 36975512 Free PMC article.
References
-
- R&D blueprints. Key Actions by Disease. Oslo: World Health Organisation; (2020).
-
- Colmenero I, Santonja C, Alonso-Riano M, Noguera-Morel L, Hernandez-Martin A, Andina D, et al. . SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. (2020) 183:729–37. 10.1111/bjd.19327 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

