Better Together: Current Insights Into Phagosome-Lysosome Fusion
- PMID: 33717183
- PMCID: PMC7946854
- DOI: 10.3389/fimmu.2021.636078
Better Together: Current Insights Into Phagosome-Lysosome Fusion
Abstract
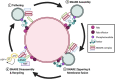
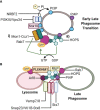
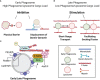
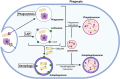
Following phagocytosis, the nascent phagosome undergoes maturation to become a phagolysosome with an acidic, hydrolytic, and often oxidative lumen that can efficiently kill and digest engulfed microbes, cells, and debris. The fusion of phagosomes with lysosomes is a principal driver of phagosomal maturation and is targeted by several adapted intracellular pathogens. Impairment of this process has significant consequences for microbial infection, tissue inflammation, the onset of adaptive immunity, and disease. Given the importance of phagosome-lysosome fusion to phagocyte function and the many virulence factors that target it, it is unsurprising that multiple molecular pathways have evolved to mediate this essential process. While the full range of these pathways has yet to be fully characterized, several pathways involving proteins such as members of the Rab GTPases, tethering factors and SNAREs have been identified. Here, we summarize the current state of knowledge to clarify the ambiguities in the field and construct a more comprehensive phagolysosome formation model. Lastly, we discuss how other cellular pathways help support phagolysosome biogenesis and, consequently, phagocyte function.
Keywords: homeostasis; lysosome; membrane fusion; microbial clearance; phagocyte; phagosome; phagosome maturation; phagosome-lysosome fusion.
Copyright © 2021 Nguyen and Yates.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
A guide to measuring phagosomal dynamics.FEBS J. 2021 Mar;288(5):1412-1433. doi: 10.1111/febs.15506. Epub 2020 Aug 16. FEBS J. 2021. PMID: 32757358 Free PMC article. Review.
-
Sequential action of Caenorhabditis elegans Rab GTPases regulates phagolysosome formation during apoptotic cell degradation.Proc Natl Acad Sci U S A. 2010 Oct 19;107(42):18016-21. doi: 10.1073/pnas.1008946107. Epub 2010 Oct 4. Proc Natl Acad Sci U S A. 2010. PMID: 20921409 Free PMC article.
-
How nascent phagosomes mature to become phagolysosomes.Trends Immunol. 2012 Aug;33(8):397-405. doi: 10.1016/j.it.2012.03.003. Epub 2012 May 3. Trends Immunol. 2012. PMID: 22560866 Review.
-
Biochemically Reconstituted Fusion of Phagosomes with Endosomes and Lysosomes.Methods Mol Biol. 2023;2692:247-259. doi: 10.1007/978-1-0716-3338-0_17. Methods Mol Biol. 2023. PMID: 37365473
-
Size-dependent mechanism of cargo sorting during lysosome-phagosome fusion is controlled by Rab34.Proc Natl Acad Sci U S A. 2012 Dec 11;109(50):20485-90. doi: 10.1073/pnas.1206811109. Epub 2012 Nov 28. Proc Natl Acad Sci U S A. 2012. PMID: 23197834 Free PMC article.
Cited by
-
Pseudomonas aeruginosa Can Diversify after Host Cell Invasion to Establish Multiple Intracellular Niches.mBio. 2022 Dec 20;13(6):e0274222. doi: 10.1128/mbio.02742-22. Epub 2022 Nov 14. mBio. 2022. PMID: 36374039 Free PMC article.
-
Endocytosis and Alzheimer's disease.Geroscience. 2024 Feb;46(1):71-85. doi: 10.1007/s11357-023-00923-1. Epub 2023 Aug 30. Geroscience. 2024. PMID: 37646904 Free PMC article. Review.
-
Combined regulation of pro-inflammatory cytokines production by STAT3 and STAT5 in a model of B. pertussis infection of alveolar macrophages.Front Immunol. 2023 Sep 28;14:1254276. doi: 10.3389/fimmu.2023.1254276. eCollection 2023. Front Immunol. 2023. PMID: 37841236 Free PMC article.
-
The expanding boundaries of sphingolipid lysosomal storage diseases; insights from Niemann-Pick disease type C.Biochem Soc Trans. 2023 Oct 31;51(5):1777-1787. doi: 10.1042/BST20220711. Biochem Soc Trans. 2023. PMID: 37844193 Free PMC article. Review.
-
Replicative Acinetobacter baumannii strains interfere with phagosomal maturation by modulating the vacuolar pH.bioRxiv [Preprint]. 2023 Feb 2:2023.02.02.526753. doi: 10.1101/2023.02.02.526753. bioRxiv. 2023. Update in: PLoS Pathog. 2023 Jun 9;19(6):e1011173. doi: 10.1371/journal.ppat.1011173. PMID: 36778331 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

