ZINC40099027 activates human focal adhesion kinase by accelerating the enzymatic activity of the FAK kinase domain
- PMID: 33715263
- PMCID: PMC7955952
- DOI: 10.1002/prp2.737
ZINC40099027 activates human focal adhesion kinase by accelerating the enzymatic activity of the FAK kinase domain
Abstract
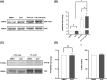
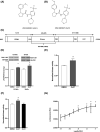
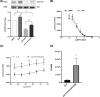
Focal adhesion kinase (FAK) regulates gastrointestinal epithelial restitution and healing. ZINC40099027 (Zn27) activates cellular FAK and promotes intestinal epithelial wound closure in vitro and in mice. However, whether Zn27 activates FAK directly or indirectly remains unknown. We evaluated Zn27 potential modulation of the key phosphatases, PTP-PEST, PTP1B, and SHP2, that inactivate FAK, and performed in vitro kinase assays with purified FAK to assess direct Zn27-FAK interaction. In human Caco-2 cells, Zn27-stimulated FAK-Tyr-397 phosphorylation despite PTP-PEST inhibition and did not affect PTP1B-FAK interaction or SHP2 activity. Conversely, in vitro kinase assays demonstrated that Zn27 directly activates both full-length 125 kDa FAK and its 35 kDa kinase domain. The ATP-competitive FAK inhibitor PF573228 reduced basal and ZN27-stimulated FAK phosphorylation in Caco-2 cells, but Zn27 increased FAK phosphorylation even in cells treated with PF573228. Increasing PF573228 concentrations completely prevented activation of 35 kDa FAK in vitro by a normally effective Zn27 concentration. Conversely, increasing Zn27 concentrations dose-dependently activated kinase activity and overcame PF573228 inhibition of FAK, suggesting the direct interactions of Zn27 with FAK may be competitive. Zn27 increased the maximal activity (Vmax ) of FAK. The apparent Km of the substrate also increased under laboratory conditions less relevant to intracellular ATP concentrations. These results suggest that Zn27 is highly potent and enhances FAK activity via allosteric interaction with the FAK kinase domain to increase the Vmax of FAK for ATP. Understanding Zn27 enhancement of FAK activity will be important to redesign and develop a clinical drug that can promote mucosal wound healing.
Keywords: focal adhesion kinase; migration; mucosal repair; nonsteroidal anti-inflammatory drugs.
© 2021 The Authors. Pharmacology Research & Perspectives published by John Wiley & Sons Ltd, British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Enhanced Bone Regeneration through Regulation of Mechanoresponsive FAK-ERK1/2 Signaling by ZINC40099027 during Distraction Osteogenesis.Int J Med Sci. 2024 Jan 1;21(1):137-150. doi: 10.7150/ijms.88298. eCollection 2024. Int J Med Sci. 2024. PMID: 38164350 Free PMC article.
-
ZINC40099027 Promotes Gastric Mucosal Repair in Ongoing Aspirin-Associated Gastric Injury by Activating Focal Adhesion Kinase.Cells. 2021 Apr 15;10(4):908. doi: 10.3390/cells10040908. Cells. 2021. PMID: 33920786 Free PMC article.
-
Small molecule FAK activator promotes human intestinal epithelial monolayer wound closure and mouse ulcer healing.Sci Rep. 2019 Oct 11;9(1):14669. doi: 10.1038/s41598-019-51183-z. Sci Rep. 2019. PMID: 31604999 Free PMC article.
-
ZINC40099027 promotes monolayer circular defect closure by a novel pathway involving cytosolic activation of focal adhesion kinase and downstream paxillin and ERK1/2.Cell Tissue Res. 2022 Nov;390(2):261-279. doi: 10.1007/s00441-022-03674-1. Epub 2022 Aug 24. Cell Tissue Res. 2022. PMID: 36001146
-
Repetitive deformation activates Src-independent FAK-dependent ERK motogenic signals in human Caco-2 intestinal epithelial cells.Am J Physiol Cell Physiol. 2008 Jun;294(6):C1350-61. doi: 10.1152/ajpcell.00027.2008. Epub 2008 Apr 9. Am J Physiol Cell Physiol. 2008. PMID: 18400991 Free PMC article.
Cited by
-
Enhanced Bone Regeneration through Regulation of Mechanoresponsive FAK-ERK1/2 Signaling by ZINC40099027 during Distraction Osteogenesis.Int J Med Sci. 2024 Jan 1;21(1):137-150. doi: 10.7150/ijms.88298. eCollection 2024. Int J Med Sci. 2024. PMID: 38164350 Free PMC article.
-
ZINC40099027 Promotes Gastric Mucosal Repair in Ongoing Aspirin-Associated Gastric Injury by Activating Focal Adhesion Kinase.Cells. 2021 Apr 15;10(4):908. doi: 10.3390/cells10040908. Cells. 2021. PMID: 33920786 Free PMC article.
-
Force-dependent focal adhesion assembly and disassembly: A computational study.PLoS Comput Biol. 2023 Oct 6;19(10):e1011500. doi: 10.1371/journal.pcbi.1011500. eCollection 2023 Oct. PLoS Comput Biol. 2023. PMID: 37801464 Free PMC article.
-
Sustained intestinal epithelial monolayer wound closure after transient application of a FAK-activating small molecule.PLoS One. 2024 Aug 16;19(8):e0304010. doi: 10.1371/journal.pone.0304010. eCollection 2024. PLoS One. 2024. PMID: 39150901 Free PMC article.
-
A novel drug-like water-soluble small molecule Focal Adhesion Kinase (FAK) activator promotes intestinal mucosal healing.Curr Res Pharmacol Drug Discov. 2022 Dec 23;4:100147. doi: 10.1016/j.crphar.2022.100147. eCollection 2023. Curr Res Pharmacol Drug Discov. 2022. PMID: 36632414 Free PMC article.
References
-
- Froslie, KF , Jahnsen, J , Moum, BA , Vatn, MH ; IBSEN Group . Mucosal healing in inflammatory bowel disease: results from a Norwegian population‐based cohort. Gastroenterology. 2007;133:412‐422. - PubMed
-
- Basson MD. Hierarchies of healing in gut mucosal injury. J Physiol Pharmacol. 2017;68(6):789‐795. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

