Dual-specificity phosphatase 3 deletion promotes obesity, non-alcoholic steatohepatitis and hepatocellular carcinoma
- PMID: 33712680
- PMCID: PMC7954796
- DOI: 10.1038/s41598-021-85089-6
Dual-specificity phosphatase 3 deletion promotes obesity, non-alcoholic steatohepatitis and hepatocellular carcinoma
Abstract
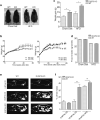
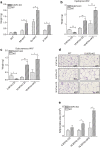
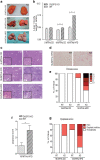
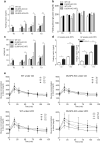
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic hepatic pathology in Western countries. It encompasses a spectrum of conditions ranging from simple steatosis to more severe and progressive non-alcoholic steatohepatitis (NASH) that can lead to hepatocellular carcinoma (HCC). Obesity and related metabolic syndrome are important risk factors for the development of NAFLD, NASH and HCC. DUSP3 is a small dual-specificity protein phosphatase with a poorly known physiological function. We investigated its role in metabolic syndrome manifestations and in HCC using a mouse knockout (KO) model. While aging, DUSP3-KO mice became obese, exhibited insulin resistance, NAFLD and associated liver damage. These phenotypes were exacerbated under high fat diet (HFD). In addition, DEN administration combined to HFD led to rapid HCC development in DUSP3-KO compared to wild type (WT) mice. DUSP3-KO mice had more serum triglycerides, cholesterol, AST and ALT compared to control WT mice under both regular chow diet (CD) and HFD. The level of fasting insulin was higher compared to WT mice, though, fasting glucose as well as glucose tolerance were normal. At the molecular level, HFD led to decreased expression of DUSP3 in WT mice. DUSP3 deletion was associated with increased and consistent phosphorylation of the insulin receptor (IR) and with higher activation of the downstream signaling pathway. In conclusion, our results support a new role for DUSP3 in obesity, insulin resistance, NAFLD and liver damage.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Degradation of PHLPP2 by KCTD17, via a Glucagon-Dependent Pathway, Promotes Hepatic Steatosis.Gastroenterology. 2017 Dec;153(6):1568-1580.e10. doi: 10.1053/j.gastro.2017.08.039. Epub 2017 Aug 30. Gastroenterology. 2017. PMID: 28859855 Free PMC article.
-
MicroRNA-223 Ameliorates Nonalcoholic Steatohepatitis and Cancer by Targeting Multiple Inflammatory and Oncogenic Genes in Hepatocytes.Hepatology. 2019 Oct;70(4):1150-1167. doi: 10.1002/hep.30645. Epub 2019 Jun 5. Hepatology. 2019. PMID: 30964207 Free PMC article.
-
miR-21-5p promotes NASH-related hepatocarcinogenesis.Liver Int. 2023 Oct;43(10):2256-2274. doi: 10.1111/liv.15682. Epub 2023 Aug 3. Liver Int. 2023. PMID: 37534739
-
Nonalcoholic fatty liver disease and hepatocellular carcinoma.Metabolism. 2016 Aug;65(8):1151-60. doi: 10.1016/j.metabol.2016.01.010. Epub 2016 Jan 23. Metabolism. 2016. PMID: 26907206 Review.
-
Non-alcoholic fatty liver disease associated with hepatocellular carcinoma: An increasing concern.Indian J Med Res. 2019 Jan;149(1):9-17. doi: 10.4103/ijmr.IJMR_1456_17. Indian J Med Res. 2019. PMID: 31115369 Free PMC article. Review.
Cited by
-
DUSP3 regulates phosphorylation-mediated degradation of occludin and is required for maintaining epithelial tight junction.J Biomed Sci. 2022 Jun 15;29(1):40. doi: 10.1186/s12929-022-00826-x. J Biomed Sci. 2022. PMID: 35705979 Free PMC article.
-
Targeting Protein Phosphatases for the Treatment of Chronic Liver Disease.Curr Drug Targets. 2024;25(3):171-189. doi: 10.2174/0113894501278886231221092522. Curr Drug Targets. 2024. PMID: 38213163 Review.
-
Hepatocyte phosphatase DUSP22 mitigates NASH-HCC progression by targeting FAK.Nat Commun. 2022 Oct 8;13(1):5945. doi: 10.1038/s41467-022-33493-5. Nat Commun. 2022. PMID: 36209205 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

