Peptide Hormone Insulin Regulates Function, Expression, and SUMOylation of Organic Anion Transporter 3
- PMID: 33709304
- PMCID: PMC9536509
- DOI: 10.1208/s12248-021-00575-z
Peptide Hormone Insulin Regulates Function, Expression, and SUMOylation of Organic Anion Transporter 3
Abstract
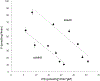
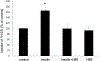
Organic anion transporter 3 (OAT3) plays an important role in the disposition of various anionic drugs which impacts the pharmacokinetics and pharmacodynamics of the therapeutics, thus influencing the pharmacological effects and toxicity of the drugs. In this study, we investigated the effect of insulin on the regulation of OAT3 function, expression, and SUMOylation. We demonstrated that insulin induced an increase in OAT3 transport activity through a dose- and time-dependent manner in COS-7 cells. The insulin-induced elevation in OAT3 function was blocked by PKA inhibitor H89, which correlated well with OAT3 protein expression. Moreover, both PKA activator Bt2-cAMP-induced increase and insulin-induced increase in OAT3 function were blocked by PKB inhibitor AKTi1/2. To further investigate the involvement of SUMOylation, we treated OAT3-expressing cells with insulin in presence or absence of H89 or AKTi1/2 followed by examining OAT3 SUMOylation. We showed that insulin enhanced OAT3 SUMOylation, and such enhancement was abrogated by H89 and AKTi1/2. Lastly, insulin increased OAT3 function and SUMOylation in rat kidney slice. In conclusion, our investigations demonstrated that insulin regulated OAT3 function, expression, and SUMOylation through PKA/PKB signaling pathway. Graphical abstract.
Keywords: Drug transport; Insulin; Organic anion transporter 3; Regulation; SUMOylation.
Conflict of interest statement
Conflict of interest
The authors have declared that there is no conflict of interest.
Figures
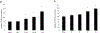
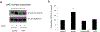
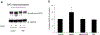
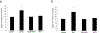
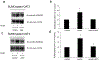
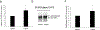
Similar articles
-
Activation of Protein Kinase A Stimulates SUMOylation, Expression, and Transport Activity of Organic Anion Transporter 3.AAPS J. 2019 Feb 13;21(2):30. doi: 10.1208/s12248-019-0303-4. AAPS J. 2019. PMID: 30761470 Free PMC article.
-
The SUMO-Specific Protease Senp2 Regulates SUMOylation, Expression and Function of Human Organic Anion Transporter 3.Biochim Biophys Acta Biomembr. 2019 Jul 1;1861(7):1293-1301. doi: 10.1016/j.bbamem.2019.04.007. Epub 2019 May 1. Biochim Biophys Acta Biomembr. 2019. PMID: 31054272 Free PMC article.
-
Insulin-like growth factor 1 modulates the phosphorylation, expression, and activity of organic anion transporter 3 through protein kinase A signaling pathway.Acta Pharm Sin B. 2020 Jan;10(1):186-194. doi: 10.1016/j.apsb.2019.05.005. Epub 2019 Jun 5. Acta Pharm Sin B. 2020. PMID: 31993315 Free PMC article.
-
Identification and Quantitative Assessment of Uremic Solutes as Inhibitors of Renal Organic Anion Transporters, OAT1 and OAT3.Mol Pharm. 2016 Sep 6;13(9):3130-40. doi: 10.1021/acs.molpharmaceut.6b00332. Epub 2016 Aug 9. Mol Pharm. 2016. PMID: 27467266 Review.
-
Recent Advances in Synthetic Drugs and Natural Actives Interacting with OAT3.Molecules. 2023 Jun 13;28(12):4740. doi: 10.3390/molecules28124740. Molecules. 2023. PMID: 37375294 Free PMC article. Review.
Cited by
-
Pancreatic Hormone Insulin Modulates Organic Anion Transporter 1 in the Kidney: Regulation via Remote Sensing and Signaling Network.AAPS J. 2023 Jan 10;25(1):13. doi: 10.1208/s12248-022-00778-y. AAPS J. 2023. PMID: 36627500 Free PMC article.
-
The regulation of human organic anion transporter 4 by insulin-like growth factor 1 and protein kinase B signaling.Biochem Pharmacol. 2023 Sep;215:115702. doi: 10.1016/j.bcp.2023.115702. Epub 2023 Jul 23. Biochem Pharmacol. 2023. PMID: 37487877 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

