A Human-Based Functional NMJ System for Personalized ALS Modeling and Drug Testing
- PMID: 33709015
- PMCID: PMC7942691
- DOI: 10.1002/adtp.202000133
A Human-Based Functional NMJ System for Personalized ALS Modeling and Drug Testing
Abstract
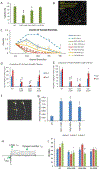
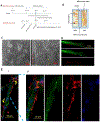
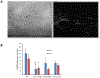
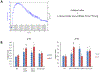
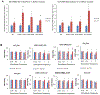
Loss of the neuromuscular junction (NMJ) is an early and critical hallmark in all forms of ALS. The study design was to develop a functional NMJ disease model by integrating motoneurons (MNs) differentiated from multiple ALS-patients' induced pluripotent stem cells (iPSCs) and primary human muscle into a chambered system. NMJ functionality was tested by recording myotube contractions while stimulating MNs by field electrodes and a set of clinically relevant parameters were defined to characterize the NMJ function. Three ALS lines were analyzed, 2 with SOD1 mutations and 1 with a FUS mutation. The ALS-MNs reproduced pathological phenotypes, including increased axonal varicosities, reduced axonal branching and elongation and increased excitability. These MNs formed functional NMJs with wild type muscle, but with significant deficits in NMJ quantity, fidelity and fatigue index. Furthermore, treatment with the Deana protocol was found to correct the NMJ deficits in all the ALS mutant lines tested. Quantitative analysis also revealed the variations inherent in each mutant lines. This functional NMJ system provides a platform for the study of both fALS and sALS and has the capability of being adapted into subtype-specific or patient-specific models for ALS etiological investigation and patient stratification for drug testing.
Keywords: ALS; functional model; human-based; neuromuscular junction; patient-specific.
Figures



Similar articles
-
Optimization of Application-Driven Development of In Vitro Neuromuscular Junction Models.Tissue Eng Part B Rev. 2022 Dec;28(6):1180-1191. doi: 10.1089/ten.TEB.2021.0204. Epub 2022 Aug 1. Tissue Eng Part B Rev. 2022. PMID: 35018825 Free PMC article. Review.
-
ALS mutations in both human skeletal muscle and motoneurons differentially affects neuromuscular junction integrity and function.Biomaterials. 2022 Oct;289:121752. doi: 10.1016/j.biomaterials.2022.121752. Epub 2022 Aug 19. Biomaterials. 2022. PMID: 36084484
-
The Effect of Skeletal Muscle-Specific Creatine Treatment on ALS NMJ Integrity and Function.Int J Mol Sci. 2023 Aug 31;24(17):13519. doi: 10.3390/ijms241713519. Int J Mol Sci. 2023. PMID: 37686322 Free PMC article.
-
Properties of Glial Cell at the Neuromuscular Junction Are Incompatible with Synaptic Repair in the SOD1G37R ALS Mouse Model.J Neurosci. 2020 Sep 30;40(40):7759-7777. doi: 10.1523/JNEUROSCI.1748-18.2020. Epub 2020 Aug 28. J Neurosci. 2020. PMID: 32859714 Free PMC article.
-
Small junction, big problems: Neuromuscular junction pathology in mouse models of amyotrophic lateral sclerosis (ALS).J Anat. 2022 Nov;241(5):1089-1107. doi: 10.1111/joa.13463. Epub 2021 Jun 7. J Anat. 2022. PMID: 34101196 Free PMC article. Review.
Cited by
-
Optimization of Application-Driven Development of In Vitro Neuromuscular Junction Models.Tissue Eng Part B Rev. 2022 Dec;28(6):1180-1191. doi: 10.1089/ten.TEB.2021.0204. Epub 2022 Aug 1. Tissue Eng Part B Rev. 2022. PMID: 35018825 Free PMC article. Review.
-
The Evolution of Complex Muscle Cell In Vitro Models to Study Pathomechanisms and Drug Development of Neuromuscular Disease.Cells. 2022 Apr 5;11(7):1233. doi: 10.3390/cells11071233. Cells. 2022. PMID: 35406795 Free PMC article. Review.
-
A Functional Human-on-a-Chip Autoimmune Disease Model of Myasthenia Gravis for Development of Therapeutics.Front Cell Dev Biol. 2021 Nov 22;9:745897. doi: 10.3389/fcell.2021.745897. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34881241 Free PMC article.
-
Unlocking the Complexity of Neuromuscular Diseases: Insights from Human Pluripotent Stem Cell-Derived Neuromuscular Junctions.Int J Mol Sci. 2023 Oct 18;24(20):15291. doi: 10.3390/ijms242015291. Int J Mol Sci. 2023. PMID: 37894969 Free PMC article. Review.
-
Early Alterations in Structural and Functional Properties in the Neuromuscular Junctions of Mutant FUS Mice.Int J Mol Sci. 2023 May 19;24(10):9022. doi: 10.3390/ijms24109022. Int J Mol Sci. 2023. PMID: 37240370 Free PMC article.
References
-
- Ticozzi N, and Silani V, in Neurodegenerative Diseases: Clinical Aspects, Molecular Genetics and Biomarkers, Vol ;
- Beghi E, Mennini T, Bendotti C, Bigini P, Logroscino G, Chio A, Hardiman O, Mitchell D, Swingler R, Traynor BJ, and Al-Chalabi A, Curr. Med. Chem. 2007, 14, 3185–200. - PubMed
-
- Serio A, and Patani R, Stem Cells 2018, 36, 293–303; - PubMed
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, D. H., Takahashi R, Misawa H, and Cleveland DW, Nat. Neurosci. 2008, 11, 251–253; - PMC - PubMed
- Frakes Ashley E., Ferraiuolo L, Haidet-Phillips Amanda M., Schmelzer L, Braun L, Miranda Carlos J., Ladner Katherine J., Bevan Adam K., Foust Kevin D., Godbout Jonathan P., Popovich Phillip G., Guttridge Denis C., and Kaspar Brian K., Neuron 81, 1009–1023. - PMC - PubMed
-
- Sabatelli M, Conte A, and Zollino M, Clin. Genet. 2013, 83, 408–416. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
