The role of intellectual property rights on access to medicines in the WHO African region: 25 years after the TRIPS agreement
- PMID: 33706726
- PMCID: PMC7951129
- DOI: 10.1186/s12889-021-10374-y
The role of intellectual property rights on access to medicines in the WHO African region: 25 years after the TRIPS agreement
Abstract
Background: It is now 25 years since the adoption of the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) and the same concerns raised during its negotiations such as high prices of medicines, market exclusivity and delayed market entry for generics remain relevant as highlighted recently by the Ebola and COVID-19 pandemics. The World Health Organization's (WHO) mandate to work on the interface between intellectual property, innovation and access to medicine has been continually reinforced and extended to include providing support to countries on the implementation of TRIPS flexibilities in collaboration with stakeholders. This study analyses the role of intellectual property on access to medicines in the African Region.
Methods: We analyze patent data from the African Regional Intellectual Property Organization (ARIPO) and Organisation Africaine de la Propriété Intellectuelle (OAPI) to provide a situational analysis of patenting activity and trends. We also review legislation to assess how TRIPS flexibilities are implemented in countries.
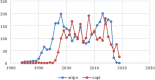
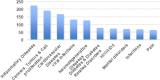
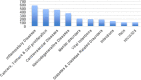
Results: Patenting was low for African countries. Only South Africa and Cameroon appeared in the list of top ten originator countries for ARIPO and OAPI respectively. Main diseases covered by African patents were HIV/AIDS, cardiovascular diseases, cancers and tumors. Majority countries have legislation allowing for compulsory licensing and parallel importation of medicines, while the least legislated flexibilities were explicit exemption of pharmaceutical products from patentable subject matter, new or second use of patented pharmaceutical products, imposition of limits to patent term extension and test data protection. Thirty-nine countries have applied TRIPS flexibilities, with the most common being compulsory licensing and least developed country transition provisions.
Conclusions: Opportunities exist for WHO to work with ARIPO and OAPI to support countries in reviewing their legislation to be more responsive to public health needs.
Keywords: Access to medicinal products; African region; Intellectual property; TRIPS flexibilities; WHO.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Has the implementation of the TRIPS Agreement in Latin America and the Caribbean produced intellectual property legislation that favours public health?Bull World Health Organ. 2004 Nov;82(11):815-21. Epub 2004 Dec 14. Bull World Health Organ. 2004. PMID: 15640916 Free PMC article.
-
What is the impact of intellectual property rules on access to medicines? A systematic review.Global Health. 2022 Apr 15;18(1):40. doi: 10.1186/s12992-022-00826-4. Global Health. 2022. PMID: 35428250 Free PMC article. Review.
-
Medicine procurement and the use of flexibilities in the Agreement on Trade-Related Aspects of Intellectual Property Rights, 2001-2016.Bull World Health Organ. 2018 Mar 1;96(3):185-193. doi: 10.2471/BLT.17.199364. Epub 2018 Feb 5. Bull World Health Organ. 2018. PMID: 29531417 Free PMC article.
-
Public health-oriented intellectual property and trade policies in Africa and the regional mechanism under Trade-Related Aspects of Intellectual Property Rights amendment.Public Health. 2019 Aug;173:1-4. doi: 10.1016/j.puhe.2019.04.019. Epub 2019 Jun 13. Public Health. 2019. PMID: 31203136
-
[Evolution of the international intellectual property rights system: patent protection for the pharmaceutical industry and access to medicines].Cad Saude Publica. 2007 Feb;23(2):257-67. doi: 10.1590/s0102-311x2007000200002. Cad Saude Publica. 2007. PMID: 17221075 Review. Portuguese.
Cited by
-
A Pandemic Instrument can Optimize the Regime Complex for AMR by Striking a Balance between Centralization and Decentralization.J Law Med Ethics. 2022;50(S2):26-33. doi: 10.1017/jme.2022.76. J Law Med Ethics. 2022. PMID: 36889353 Free PMC article.
-
A comprehensive framework identifying barriers to global health R&D innovation and access.BMJ Glob Health. 2023 Sep;8(9):e013076. doi: 10.1136/bmjgh-2023-013076. BMJ Glob Health. 2023. PMID: 37751936 Free PMC article. Review.
-
Exploring SureChEMBL from a drug discovery perspective.Sci Data. 2024 May 16;11(1):507. doi: 10.1038/s41597-024-03371-4. Sci Data. 2024. PMID: 38755219 Free PMC article.
-
Combining legal epidemiology and implementation science to improve global access to medicines: challenges and opportunities.Front Health Serv. 2024 Jan 9;3:1291183. doi: 10.3389/frhs.2023.1291183. eCollection 2023. Front Health Serv. 2024. PMID: 38264186 Free PMC article.
-
TRIPS to Where? A Narrative Review of the Empirical Literature on Intellectual Property Licensing Models to Promote Global Diffusion of Essential Medicines.Pharmaceutics. 2021 Dec 27;14(1):48. doi: 10.3390/pharmaceutics14010048. Pharmaceutics. 2021. PMID: 35056944 Free PMC article. Review.
References
-
- World Trade Organization (WTO), Agreement on Trade-Related Aspects of Intellectual Property Rights, Apr. 15, 1994, Marrakesh Agreement Establishing the World Trade Organization, Annex 1C, 1869 U.N.T.S. 299, 33 I.L.M. 1197 (1994).
-
- Ferreira LLG, Andriacopulo AD. Drugs and Vaccines in the 21st Century for Neglected Diseases. Lancet/Infections. 2019;19:125–126. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical