mtDNA Heteroplasmy at the Core of Aging-Associated Heart Failure. An Integrative View of OXPHOS and Mitochondrial Life Cycle in Cardiac Mitochondrial Physiology
- PMID: 33692999
- PMCID: PMC7937615
- DOI: 10.3389/fcell.2021.625020
mtDNA Heteroplasmy at the Core of Aging-Associated Heart Failure. An Integrative View of OXPHOS and Mitochondrial Life Cycle in Cardiac Mitochondrial Physiology
Abstract
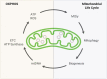
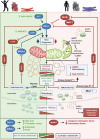
The most common aging-associated diseases are cardiovascular diseases which affect 40% of elderly people. Elderly people are prone to suffer aging-associated diseases which are not only related to health and medical cost but also to labor, household productivity and mortality cost. Aging is becoming a world problem and it is estimated that 21.8% of global population will be older than 65 years old in 2050; and for the first time in human history, there will be more elderly people than children. It is well accepted that the origin of aging-associated cardiovascular diseases is mitochondrial dysfunction. Mitochondria have their own genome (mtDNA) that is circular, double-stranded, and 16,569 bp long in humans. There are between 500 to 6000 mtDNA copies per cell which are tissue-specific. As a by-product of ATP production, reactive oxygen species (ROS) are generated which damage proteins, lipids, and mtDNA. ROS-mutated mtDNA co-existing with wild type mtDNA is called mtDNA heteroplasmy. The progressive increase in mtDNA heteroplasmy causes progressive mitochondrial dysfunction leading to a loss in their bioenergetic capacity, disruption in the balance of mitochondrial fusion and fission events (mitochondrial dynamics, MtDy) and decreased mitophagy. This failure in mitochondrial physiology leads to the accumulation of depolarized and ROS-generating mitochondria. Thus, besides attenuated ATP production, dysfunctional mitochondria interfere with proper cellular metabolism and signaling pathways in cardiac cells, contributing to the development of aging-associated cardiovascular diseases. In this context, there is a growing interest to enhance mitochondrial function by decreasing mtDNA heteroplasmy. Reduction in mtDNA heteroplasmy is associated with increased mitophagy, proper MtDy balance and mitochondrial biogenesis; and those processes can delay the onset or progression of cardiovascular diseases. This has led to the development of mitochondrial therapies based on the application of nutritional, pharmacological and genetic treatments. Those seeking to have a positive impact on mtDNA integrity, mitochondrial biogenesis, dynamics and mitophagy in old and sick hearts. This review covers the current knowledge of mitochondrial physiopathology in aging, how disruption of OXPHOS or mitochondrial life cycle alter mtDNA and cardiac cell function; and novel mitochondrial therapies to protect and rescue our heart from cardiovascular diseases.
Keywords: OXPHOS; ROS; aging; biogenesis; cardiac; heart failure; mitophagy; mtDNA heteroplasmy.
Copyright © 2021 Elorza and Soffia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Mitochondrial biogenesis: pharmacological approaches.Curr Pharm Des. 2014;20(35):5507-9. doi: 10.2174/138161282035140911142118. Curr Pharm Des. 2014. PMID: 24606795
-
Mitochondrial epigenetics in aging and cardiovascular diseases.Front Cardiovasc Med. 2023 Jul 13;10:1204483. doi: 10.3389/fcvm.2023.1204483. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37522089 Free PMC article. Review.
-
Targeting Mitochondrial Function with Chemoptogenetics.Biomedicines. 2022 Oct 1;10(10):2459. doi: 10.3390/biomedicines10102459. Biomedicines. 2022. PMID: 36289721 Free PMC article. Review.
-
Mitochondria: Biogenesis and mitophagy balance in segregation and clonal expansion of mitochondrial DNA mutations.Int J Biochem Cell Biol. 2015 Jun;63:21-4. doi: 10.1016/j.biocel.2015.01.023. Epub 2015 Feb 7. Int J Biochem Cell Biol. 2015. PMID: 25666555 Review.
-
BNIP3L/NIX and FUNDC1-mediated mitophagy is required for mitochondrial network remodeling during cardiac progenitor cell differentiation.Autophagy. 2019 Jul;15(7):1182-1198. doi: 10.1080/15548627.2019.1580095. Epub 2019 Feb 22. Autophagy. 2019. PMID: 30741592 Free PMC article.
Cited by
-
N-acetylcysteine, a small molecule scavenger of reactive oxygen species, alleviates cardiomyocyte damage by regulating OPA1-mediated mitochondrial quality control and apoptosis in response to oxidative stress.J Thorac Dis. 2024 Aug 31;16(8):5323-5336. doi: 10.21037/jtd-24-927. Epub 2024 Aug 21. J Thorac Dis. 2024. PMID: 39268103 Free PMC article.
-
Functional remodelling of perinuclear mitochondria alters nucleoplasmic Ca2+ signalling in heart failure.Philos Trans R Soc Lond B Biol Sci. 2022 Nov 21;377(1864):20210320. doi: 10.1098/rstb.2021.0320. Epub 2022 Oct 3. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 36189813 Free PMC article.
-
Vitamin C as Scavenger of Reactive Oxygen Species during Healing after Myocardial Infarction.Int J Mol Sci. 2024 Mar 7;25(6):3114. doi: 10.3390/ijms25063114. Int J Mol Sci. 2024. PMID: 38542087 Free PMC article. Review.
-
Human induced pluripotent stem cells for studying mitochondrial diseases in the heart.FEBS Lett. 2022 Jul;596(14):1735-1745. doi: 10.1002/1873-3468.14444. Epub 2022 Jul 11. FEBS Lett. 2022. PMID: 35788991 Free PMC article. Review.
-
Phenotypic Variability of Andersen-Tawil Syndrome Due to Allelic Mutation c.652C>T in the KCNJ2 Gene-A New Family Case Report.Biomolecules. 2024 Apr 22;14(4):507. doi: 10.3390/biom14040507. Biomolecules. 2024. PMID: 38672523 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

