An Antibody-Drug Conjugate That Selectively Targets Human Monocyte Progenitors for Anti-Cancer Therapy
- PMID: 33692791
- PMCID: PMC7937628
- DOI: 10.3389/fimmu.2021.618081
An Antibody-Drug Conjugate That Selectively Targets Human Monocyte Progenitors for Anti-Cancer Therapy
Abstract
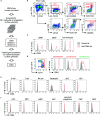
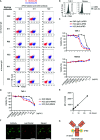
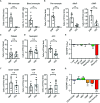
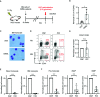
As hematopoietic progenitors supply a large number of blood cells, therapeutic strategies targeting hematopoietic progenitors are potentially beneficial to eliminate unwanted blood cells, such as leukemic cells and immune cells causing diseases. However, due to their pluripotency, targeting those cells may impair the production of multiple cell lineages, leading to serious side effects such as anemia and increased susceptibility to infection. To minimize those side effects, it is important to identify monopotent progenitors that give rise to a particular cell lineage. Monocytes and monocyte-derived macrophages play important roles in the development of inflammatory diseases and tumors. Recently, we identified human monocyte-restricted progenitors, namely, common monocyte progenitors and pre-monocytes, both of which express high levels of CD64, a well-known monocyte marker. Here, we introduce a dimeric pyrrolobenzodiazepine (dPBD)-conjugated anti-CD64 antibody (anti-CD64-dPBD) that selectively induces the apoptosis of proliferating human monocyte-restricted progenitors but not non-proliferating mature monocytes. Treatment with anti-CD64-dPBD did not affect other types of hematopoietic cells including hematopoietic stem and progenitor cells, neutrophils, lymphocytes and platelets, suggesting that its off-target effects are negligible. In line with these findings, treatment with anti-CD64-dPBD directly killed proliferating monocytic leukemia cells and prevented monocytic leukemia cell generation from bone marrow progenitors of chronic myelomonocytic leukemia patients in a patient-derived xenograft model. Furthermore, by depleting the source of monocytes, treatment with anti-CD64-dPBD ultimately eliminated tumor-associated macrophages and significantly reduced tumor size in humanized mice bearing solid tumors. Given the selective action of anti-CD64-dPBD on proliferating monocyte progenitors and monocytic leukemia cells, it should be a promising tool to target cancers and other monocyte-related inflammatory disorders with minimal side effects on other cell lineages.
Keywords: chronic myelomonocytic leukemia; common monocyte progenitor; leukemia; monocyte; tumor-associated macrophage.
Copyright © 2021 Izumi, Kanayama, Shen, Kai, Kawamura, Akiyama, Yamamoto, Nagao, Okada, Kawamata, Toyota and Ohteki.
Conflict of interest statement
MKai and ZS are employees of Kyowa Kirin Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Human CD64-targeted non-viral siRNA delivery system for blood monocyte gene modulation.Sci Rep. 2017 Feb 7;7:42171. doi: 10.1038/srep42171. Sci Rep. 2017. PMID: 28169353 Free PMC article.
-
Identification of a Human Clonogenic Progenitor with Strict Monocyte Differentiation Potential: A Counterpart of Mouse cMoPs.Immunity. 2017 May 16;46(5):835-848.e4. doi: 10.1016/j.immuni.2017.04.019. Immunity. 2017. PMID: 28514689
-
CD64/Fc gamma RI is a granulo-monocytic lineage marker on CD34+ hematopoietic progenitor cells.Blood. 1995 May 1;85(9):2402-13. Blood. 1995. PMID: 7537112
-
Fcgamma receptor 1 (CD64), a target beyond cancer.Curr Pharm Des. 2009;15(23):2712-8. doi: 10.2174/138161209788923967. Curr Pharm Des. 2009. PMID: 19689341 Review.
-
From proliferation to proliferation: monocyte lineage comes full circle.Semin Immunopathol. 2014 Mar;36(2):137-48. doi: 10.1007/s00281-013-0409-1. Epub 2014 Jan 17. Semin Immunopathol. 2014. PMID: 24435095 Free PMC article. Review.
Cited by
-
Managing the immune microenvironment of osteosarcoma: the outlook for osteosarcoma treatment.Bone Res. 2023 Feb 27;11(1):11. doi: 10.1038/s41413-023-00246-z. Bone Res. 2023. PMID: 36849442 Free PMC article. Review.
-
Targeting the epigenome to reinvigorate T cells for cancer immunotherapy.Mil Med Res. 2023 Dec 4;10(1):59. doi: 10.1186/s40779-023-00496-2. Mil Med Res. 2023. PMID: 38044445 Free PMC article. Review.
-
Sarcopenia accompanied by systemic inflammation can predict clinical outcomes in patients with head and neck cancer undergoing curative therapy.Front Oncol. 2024 Mar 14;14:1378762. doi: 10.3389/fonc.2024.1378762. eCollection 2024. Front Oncol. 2024. PMID: 38549928 Free PMC article.
-
Gemcitabine-mediated depletion of immunosuppressive dendritic cells enhances the efficacy of therapeutic vaccination.Front Immunol. 2022 Oct 10;13:991311. doi: 10.3389/fimmu.2022.991311. eCollection 2022. Front Immunol. 2022. PMID: 36300124 Free PMC article.
-
Macrophages as determinants and regulators of fibrosis in systemic sclerosis.Rheumatology (Oxford). 2023 Feb 1;62(2):535-545. doi: 10.1093/rheumatology/keac410. Rheumatology (Oxford). 2023. PMID: 35861385 Free PMC article.
References
-
- Catalano L, Improta S, de Laurentiis M, Molica S, Majolino I, Musto P, et al. . Prognosis of chronic myelomonocytic leukemia. Haematologica (1996) 81(4):324–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

