Oncolytic Foamy Virus - generation and properties of a nonpathogenic replicating retroviral vector system that targets chronically proliferating cancer cells
- PMID: 33692205
- PMCID: PMC8139661
- DOI: 10.1128/JVI.00015-21
Oncolytic Foamy Virus - generation and properties of a nonpathogenic replicating retroviral vector system that targets chronically proliferating cancer cells
Abstract
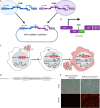
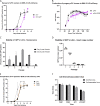
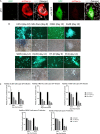
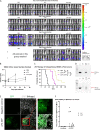
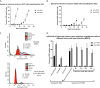
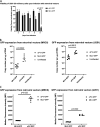
Nonpathogenic retroviruses of the Spumaretrovirinae subfamily can persist long-term in the cytoplasm of infected cells, completing their lifecycle only after the nuclear membrane dissolves at the time of cell division. Since the targeting of slowly dividing cancer cells remains an unmet need in oncolytic virotherapy we constructed a replication competent Foamy Virus vector (oFV) from the genomes of two chimpanzee Simian Foamy Viruses (PAN1 and PAN2) and inserted a GFP transgene in place of the bel-2 open reading frame. oFV-GFP infected and propagated with slow kinetics in multiple human tumor cell lines, inducing a syncytial cytopathic effect. Infection of growth arrested MRC5 cells was not productive, but oFV genomes persisted in the cytoplasm and the productive viral lifecycle resumed when cell division was later restored. In vivo, the virus propagated extensively in intraperitoneal ovarian cancer xenografts, slowing tumor growth, significantly prolonging survival of the treated mice and sustaining GFP transgene expression for at least 45 days. Our data indicate that oFV is a promising new replication-competent viral and gene delivery platform for efficient targeting of the most fundamental trait of cancer cells, their ability to sustain chronic proliferation.Significance:The infectivity of certain retroviruses is limited to dividing cells, which makes them attractive tools for targeting cancer cell proliferation. Previously developed replication-competent gammaretroviral vectors spread efficiently in rapidly dividing cancer cells, but not in cancer cells that divide more slowly. In contrast to rapidly proliferating transplantable mouse tumors, slow proliferation is a hallmark of human cancers and may have contributed to the clinical failure of the preclinically promising Murine Leukemia Virus vector Toca511 which failed to show efficacy in a phase 3 clinical trial in patients with glioblastoma. The studies presented in our manuscript show that oncolytic Foamy Virus (oFV) vectors are capable of persisting unintegrated in quiescent cells and resuming their life cycle once the cells start dividing again. This property of oFVs, together with their lack of pathogenicity and their ability to catalyze the fusion of infected cancer cells, makes them an attractive platform for further investigation.
Copyright © 2021 Budzik et al.
Figures
Similar articles
-
Evaluation of the stability and intratumoral delivery of foreign transgenes encoded by an oncolytic Foamy Virus vector.Cancer Gene Ther. 2022 Aug;29(8-9):1240-1251. doi: 10.1038/s41417-022-00431-y. Epub 2022 Feb 10. Cancer Gene Ther. 2022. PMID: 35145270 Free PMC article.
-
The efficiency of simian foamy virus vector type-1 (SFV-1) in nondividing cells and in human PBLs.Virology. 2001 Feb 15;280(2):243-52. doi: 10.1006/viro.2000.0773. Virology. 2001. PMID: 11162838
-
A Novel Chimeric Oncolytic Virus Vector for Improved Safety and Efficacy as a Platform for the Treatment of Hepatocellular Carcinoma.J Virol. 2018 Nov 12;92(23):e01386-18. doi: 10.1128/JVI.01386-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30232179 Free PMC article.
-
Beyond oncolytic virotherapy: replication-competent retrovirus vectors for selective and stable transduction of tumors.Curr Gene Ther. 2005 Dec;5(6):655-67. doi: 10.2174/156652305774964659. Curr Gene Ther. 2005. PMID: 16457654 Review.
-
Oncolytic virus therapy using genetically engineered herpes simplex viruses.Front Biosci. 2008 Jan 1;13:2060-4. doi: 10.2741/2823. Front Biosci. 2008. PMID: 17981691 Review.
Cited by
-
Plasma antibodies from humans infected with zoonotic simian foamy virus do not inhibit cell-to-cell transmission of the virus despite binding to the surface of infected cells.PLoS Pathog. 2022 May 23;18(5):e1010470. doi: 10.1371/journal.ppat.1010470. eCollection 2022 May. PLoS Pathog. 2022. PMID: 35605011 Free PMC article.
-
Sindbis Virus Vaccine Platform: A Promising Oncolytic Virus-Mediated Approach for Ovarian Cancer Treatment.Int J Mol Sci. 2024 Mar 2;25(5):2925. doi: 10.3390/ijms25052925. Int J Mol Sci. 2024. PMID: 38474178 Free PMC article. Review.
-
Tutorial: design, production and testing of oncolytic viruses for cancer immunotherapy.Nat Protoc. 2024 Sep;19(9):2540-2570. doi: 10.1038/s41596-024-00985-1. Epub 2024 May 20. Nat Protoc. 2024. PMID: 38769145 Review.
-
Determinants of Retroviral Integration and Implications for Gene Therapeutic MLV-Based Vectors and for a Cure for HIV-1 Infection.Viruses. 2022 Dec 21;15(1):32. doi: 10.3390/v15010032. Viruses. 2022. PMID: 36680071 Free PMC article. Review.
-
An Update on the Clinical Status, Challenges, and Future Directions of Oncolytic Virotherapy for Malignant Gliomas.Curr Treat Options Oncol. 2024 Jul;25(7):952-991. doi: 10.1007/s11864-024-01211-6. Epub 2024 Jun 19. Curr Treat Options Oncol. 2024. PMID: 38896326 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

