Lin28 Inhibits the Differentiation from Mouse Embryonic Stem Cells to Glial Lineage Cells through Upregulation of Yap1
- PMID: 33688355
- PMCID: PMC7920735
- DOI: 10.1155/2021/6674283
Lin28 Inhibits the Differentiation from Mouse Embryonic Stem Cells to Glial Lineage Cells through Upregulation of Yap1
Abstract
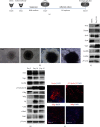
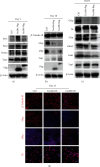
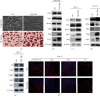
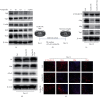
The RNA-binding protein Lin28 regulates neurogliogenesis in mammals, independently of the let-7 microRNA. However, the detailed regulatory mechanism remains obscured. Here, we established Lin28a or Lin28b overexpression mouse embryonic stem cells (ESCs) and found that these cells expressed similar levels of the core pluripotent factors, such as Oct4 and Sox2, and increased Yap1 but decreased lineage-specific markers compared to the control ESCs. Further differentiation of these ESCs to neuronal and glial lineage cells revealed that Lin28a/b overexpression did not affect the expression of neuronal marker βIII-tubulin, but dramatically inhibited the glial lineage markers, such as Gfap and Mbp. Interestingly, overexpression of Yap1 in mouse ESCs phenocopied Lin28a/b overexpression ESCs by showing defect in glial cell differentiation. Inhibition of Yap1/Tead-mediated transcription with verteporfin partially rescued the differentiation defect of Lin28a/b overexpression ESCs. Mechanistically, we demonstrated that Lin28 can directly bind to Yap1 mRNA, and the induction of Yap1 by Lin28a in mESCs is independent of Let7. Taken together, our results unravel a novel Lin28-Yap1 regulatory axis during mESC to glial lineage cell differentiation, which may shed light on glial cell generation in vitro.
Copyright © 2021 Juan Luo et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
RNA-binding protein complex LIN28/MSI2 enhances cancer stem cell-like properties by modulating Hippo-YAP1 signaling and independently of Let-7.Oncogene. 2022 Mar;41(11):1657-1672. doi: 10.1038/s41388-022-02198-w. Epub 2022 Jan 31. Oncogene. 2022. PMID: 35102250 Free PMC article.
-
Src-Yap1 signaling axis controls the trophectoderm and epiblast lineage differentiation in mouse embryonic stem cells.Stem Cell Res. 2021 Jul;54:102413. doi: 10.1016/j.scr.2021.102413. Epub 2021 May 29. Stem Cell Res. 2021. PMID: 34082184
-
MicroRNA-145 Regulates Neural Stem Cell Differentiation Through the Sox2-Lin28/let-7 Signaling Pathway.Stem Cells. 2016 May;34(5):1386-95. doi: 10.1002/stem.2309. Epub 2016 Feb 29. Stem Cells. 2016. PMID: 26849971
-
Embryonic stem cell microRNAs: defining factors in induced pluripotent (iPS) and cancer (CSC) stem cells?Curr Stem Cell Res Ther. 2009 Sep;4(3):168-77. doi: 10.2174/157488809789057400. Curr Stem Cell Res Ther. 2009. PMID: 19492978 Review.
-
The LIN28/let-7 Pathway in Cancer.Front Genet. 2017 Mar 28;8:31. doi: 10.3389/fgene.2017.00031. eCollection 2017. Front Genet. 2017. PMID: 28400788 Free PMC article. Review.
Cited by
-
RNA-binding protein complex LIN28/MSI2 enhances cancer stem cell-like properties by modulating Hippo-YAP1 signaling and independently of Let-7.Oncogene. 2022 Mar;41(11):1657-1672. doi: 10.1038/s41388-022-02198-w. Epub 2022 Jan 31. Oncogene. 2022. PMID: 35102250 Free PMC article.
-
Evolution of the Neocortex Through RNA-Binding Proteins and Post-transcriptional Regulation.Front Neurosci. 2022 Jan 10;15:803107. doi: 10.3389/fnins.2021.803107. eCollection 2021. Front Neurosci. 2022. PMID: 35082597 Free PMC article. Review.
-
LIN28A alleviates inflammation, oxidative stress, osteogenic differentiation and mineralization in lipopolysaccharide (LPS)-treated human periodontal ligament stem cells.Exp Ther Med. 2022 Jun;23(6):411. doi: 10.3892/etm.2022.11338. Epub 2022 Apr 27. Exp Ther Med. 2022. PMID: 35601075 Free PMC article.
-
Emerging roles of RNA binding proteins in intervertebral disc degeneration and osteoarthritis.Orthop Surg. 2023 Dec;15(12):3015-3025. doi: 10.1111/os.13851. Epub 2023 Oct 6. Orthop Surg. 2023. PMID: 37803912 Free PMC article. Review.
-
BACE2 variant identified from HSCR patient causes AD-like phenotypes in hPSC-derived brain organoids.Cell Death Discov. 2022 Feb 2;8(1):47. doi: 10.1038/s41420-022-00845-5. Cell Death Discov. 2022. PMID: 35110536 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

