GM-CSF Expression and Macrophage Polarization in Joints of Undifferentiated Arthritis Patients Evolving to Rheumatoid Arthritis or Psoriatic Arthritis
- PMID: 33679701
- PMCID: PMC7925849
- DOI: 10.3389/fimmu.2020.613975
GM-CSF Expression and Macrophage Polarization in Joints of Undifferentiated Arthritis Patients Evolving to Rheumatoid Arthritis or Psoriatic Arthritis
Abstract
Background and aims: GM-CSF-dependent macrophage polarization has been demonstrated in rheumatoid arthritis (RA). Our aim was to seek diagnostic/prognostic biomarkers for undifferentiated arthritis (UA) by analyzing GM-CSF expression and source, macrophage polarization and density in joints of patients with UA evolving to RA or PsA compared with established RA or PsA, respectively.
Methods: Synovial tissue (ST) from patients with UA evolving to RA (UA>RA, n=8), PsA (UA>PsA, n=9), persistent UA (UA, n=16), established RA (n=12) and PsA (n=10), and healthy controls (n=6), were analyzed. Cell source and quantitative expression of GM-CSF and proteins associated with pro-inflammatory (GM-CSF-driven) and anti-inflammatory (M-CSF-driven) macrophage polarization (activin A, TNFα, MMP12, and CD209, respectively) were assessed in ST CD163+ macrophages by multicolor immunofluorescence. GM-CSF and activin A levels were also quantified in paired synovial fluid samples. CD163+ macrophage density was determined in all groups by immunofluorescence.
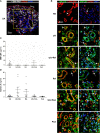
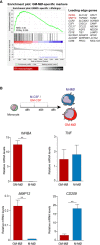
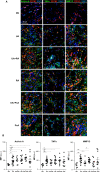
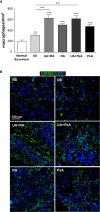
Results: Synovial stromal cells (FAP+ CD90+ fibroblast, CD90+ endothelial cells) and CD163+ sublining macrophages were the sources of GM-CSF. ST CD163+ macrophages from all groups expressed pro-inflammatory polarization markers (activin A, TNFα, and MMP12). Expression of the M-CSF-dependent anti-inflammatory marker CD209 identified two macrophage subsets (CD163+ CD209high and CD163+ CD209low/-). CD209+ macrophages were more abundant in ST from healthy controls and PsA patients, although both macrophage subtypes showed similar levels of pro-inflammatory markers in all groups. In paired synovial fluid samples, activin A was detected in all patients, with higher levels in UA>RA and RA, while GM-CSF was infrequently detected. ST CD163+ macrophage density was comparable between UA>RA and UA>PsA patients, but significantly higher than in persistent UA.
Conclusions: GM-CSF is highly expressed by sublining CD90+ FAP+ synovial fibroblasts, CD90+ activated endothelium and CD163+ macrophages in different types of arthritis. The polarization state of ST macrophages was similar in all UA and established arthritis groups, with a predominance of pro-inflammatory GM-CSF-associated markers. CD163+ macrophage density was significantly higher in the UA phases of RA and PsA compared with persistent UA. Taken together, our findings support the idea that GM-CSF is a strong driver of macrophage polarization and a potential therapeutic target not only in RA but also in PsA and all types of UA.
Keywords: GM-CSF; macrophages; psoriatic arthritis; rheumatoid arthritis; synovial tissue; undifferentiated arthritis.
Copyright © 2021 Fuentelsaz-Romero, Cuervo, Estrada-Capetillo, Celis, García-Campos, Ramírez, Sastre, Samaniego, Puig-Kröger and Cañete.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Macrophages from the synovium of active rheumatoid arthritis exhibit an activin A-dependent pro-inflammatory profile.J Pathol. 2015 Feb;235(3):515-26. doi: 10.1002/path.4466. Epub 2014 Dec 18. J Pathol. 2015. PMID: 25319955
-
Macrophage-derived cytokine and nuclear factor kappaB p65 expression in synovial membrane and skin of patients with psoriatic arthritis.Arthritis Rheum. 2000 Jun;43(6):1244-56. doi: 10.1002/1529-0131(200006)43:6<1244::AID-ANR7>3.0.CO;2-2. Arthritis Rheum. 2000. PMID: 10857783
-
The prolactin receptor is expressed in rheumatoid arthritis and psoriatic arthritis synovial tissue and contributes to macrophage activation.Rheumatology (Oxford). 2016 Dec;55(12):2248-2259. doi: 10.1093/rheumatology/kew316. Epub 2016 Sep 10. Rheumatology (Oxford). 2016. PMID: 27616146 Free PMC article.
-
Macrophages, synovial tissue and rheumatoid arthritis.Clin Exp Rheumatol. 1993 May-Jun;11(3):331-9. Clin Exp Rheumatol. 1993. PMID: 8394794 Review.
-
Synovitis in psoriatic arthritis: immunohistochemistry, comparisons with rheumatoid arthritis, and effects of therapy.Curr Rheumatol Rep. 2011 Aug;13(4):353-9. doi: 10.1007/s11926-011-0181-y. Curr Rheumatol Rep. 2011. PMID: 21503693 Free PMC article. Review.
Cited by
-
Folate Receptor Beta for Macrophage Imaging in Rheumatoid Arthritis.Front Immunol. 2022 Feb 2;13:819163. doi: 10.3389/fimmu.2022.819163. eCollection 2022. Front Immunol. 2022. PMID: 35185910 Free PMC article. Review.
-
Dendritic Cells and Macrophages in the Pathogenesis of Psoriasis.Front Immunol. 2022 Jun 28;13:941071. doi: 10.3389/fimmu.2022.941071. eCollection 2022. Front Immunol. 2022. PMID: 35837394 Free PMC article. Review.
-
Global scientific trends update on macrophage polarization in rheumatoid arthritis: A bibliometric and visualized analysis from 2000 to 2022.Heliyon. 2023 Sep 9;9(9):e19761. doi: 10.1016/j.heliyon.2023.e19761. eCollection 2023 Sep. Heliyon. 2023. PMID: 37809950 Free PMC article. Review.
-
An Overview of Growth Factors as the Potential Link between Psoriasis and Metabolic Syndrome.J Clin Med. 2023 Dec 24;13(1):109. doi: 10.3390/jcm13010109. J Clin Med. 2023. PMID: 38202116 Free PMC article. Review.
-
Macrophage colony-stimulating factor and its role in the tumor microenvironment: novel therapeutic avenues and mechanistic insights.Front Oncol. 2024 Apr 4;14:1358750. doi: 10.3389/fonc.2024.1358750. eCollection 2024. Front Oncol. 2024. PMID: 38646440 Free PMC article. Review.
References
-
- Wolfe F, Ross K, Hawley DJ, Roberts FK, Cathey MA. The prognosis of rheumatoid arthritis and undifferentiated polyarthritis syndrome in the clinic: a study of 1141 patients. J Rheumatol (1993) 20:2005–9. - PubMed
-
- Quinn MA, Green MJ, Marzo-Ortega H, Proudman S, Karim Z, Wakefield RJ, et al. . Prognostic factors in a large cohort of patients with early undifferentiated inflammatory arthritis after application of a structured management protocol. Arthritis Rheum (2003) 48:3039–45. 10.1002/art.11269 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

