A Novel Experimental Approach for In Vivo Analyses of the Salivary Gland Microvasculature
- PMID: 33679695
- PMCID: PMC7925411
- DOI: 10.3389/fimmu.2020.604470
A Novel Experimental Approach for In Vivo Analyses of the Salivary Gland Microvasculature
Abstract
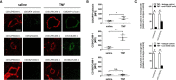
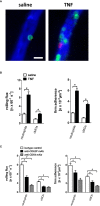
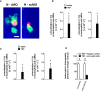
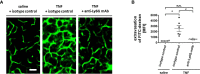
Microvascular dysfunction plays a fundamental role in the pathogenesis of salivary gland disorders. Restoring and preserving microvascular integrity might therefore represent a promising strategy for the treatment of these pathologies. The mechanisms underlying microvascular dysfunction in salivary glands, however, are still obscure, partly due to the unavailability of adequate in vivo models. Here, we present a novel experimental approach that allows comprehensive in vivo analyses of the salivary gland microvasculature in mice. For this purpose, we employed different microscopy techniques including multi-photon in vivo microscopy to quantitatively analyze interactions of distinct immune cell subsets in the submandibular gland microvasculature required for their infiltration into the surrounding parenchyma and their effects on microvascular function. Confocal microscopy and multi-channel flow cytometry in tissue sections/homogenates complemented these real-time analyses by determining the molecular phenotype of the participating cells. To this end, we identified key adhesion and signaling molecules that regulate the subset- and tissue-specific trafficking of leukocytes into inflamed glands and control the associated microvascular leakage. Hence, we established an experimental approach that allows in vivo analyses of microvascular processes in healthy and diseased salivary glands. This enables us to delineate distinct pathogenetic factors as novel therapeutic targets in salivary gland diseases.
Keywords: immunology; in vivo imaging; inflammation; leukocyte trafficking; microcirculation; microvascular permeability; salivary gland.
Copyright © 2021 Uhl, Braun, Dominik, Luft, Canis and Reichel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Plaque-associated vasa vasorum in aged apolipoprotein E-deficient mice exhibit proatherogenic functional features in vivo.Arterioscler Thromb Vasc Biol. 2013 Feb;33(2):249-56. doi: 10.1161/ATVBAHA.112.300087. Epub 2012 Dec 13. Arterioscler Thromb Vasc Biol. 2013. PMID: 23241413
-
TNF regulates leukocyte-endothelial cell interactions and microvascular dysfunction during immune complex-mediated inflammation.Br J Pharmacol. 2005 Jan;144(2):265-74. doi: 10.1038/sj.bjp.0706081. Br J Pharmacol. 2005. PMID: 15655512 Free PMC article.
-
Prenatal inflammation impairs intestinal microvascular development through a TNF-dependent mechanism and predisposes newborn mice to necrotizing enterocolitis.Am J Physiol Gastrointest Liver Physiol. 2019 Jul 1;317(1):G57-G66. doi: 10.1152/ajpgi.00332.2018. Epub 2019 May 24. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 31125264 Free PMC article.
-
Leucocyte/endothelium interactions and microvessel permeability: coupled or uncoupled?Cardiovasc Res. 2010 Jul 15;87(2):281-90. doi: 10.1093/cvr/cvq140. Epub 2010 May 13. Cardiovasc Res. 2010. PMID: 20472564 Free PMC article. Review.
-
Targeting vascular and leukocyte communication in angiogenesis, inflammation and fibrosis.Nat Rev Drug Discov. 2016 Feb;15(2):125-42. doi: 10.1038/nrd.2015.2. Epub 2015 Nov 27. Nat Rev Drug Discov. 2016. PMID: 26612664 Review.
Cited by
-
A Synopsis of Signaling Crosstalk of Pericytes and Endothelial Cells in Salivary Gland.Dent J (Basel). 2021 Dec 1;9(12):144. doi: 10.3390/dj9120144. Dent J (Basel). 2021. PMID: 34940041 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

