Human Red Blood Cells Modulate Cytokine Expression in Monocytes/Macrophages Under Anoxic Conditions
- PMID: 33679443
- PMCID: PMC7930825
- DOI: 10.3389/fphys.2021.632682
Human Red Blood Cells Modulate Cytokine Expression in Monocytes/Macrophages Under Anoxic Conditions
Abstract
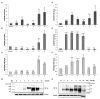
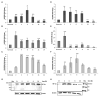
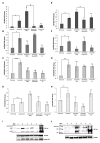
In the bone marrow (BM) hematopoietic niche, the oxygen tension is usually very low. Such condition affects stem and progenitor cell proliferation and differentiation and, at cellular level regulates hematopoietic growth factors, chemokines and adhesion molecules expression. In turn, these molecules affect the proliferation and maturation of other cellular components of the niche. Due to the complexity of the system we started the in vitro investigations of the IL-6, IL-8, TNFα cytokines expression and the vascular endothelial growth factor (VEGF), considered key mediators of the hematopoietic niche, in human macrophages and macrophage cell line. Since in the niche the oxygen availability is mediated by red blood cells (RBCs), we have influenced the anoxic cell cultures by the administration of oxygenated or deoxygenated RBCs (deoxy RBCs). The results reported in this brief paper show that the presence of RBCs up-regulates IL-8 mRNA while IL-6 and VEGF mRNA expression appears down-regulated. This does not occur when deoxy RBCs are used. Moreover, it appears that the administration of RBCs leads to an increase of TNFα expression levels in MonoMac 6 (MM6). Interestingly, the modulation of these factors likely occurs in a hypoxia-inducible factor-1α (HIF-1α) independent manner. Considering the role of oxygen in the hematopoietic niche further studies should explore these preliminary observations in more details.
Keywords: cytokine expression modulation; hypoxia; macrophages; pO2 variation; red blood cells.
Copyright © 2021 Antonelli, Scarpa and Magnani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Hematopoietic progenitor cell mobilization results in hypoxia with increased hypoxia-inducible transcription factor-1 alpha and vascular endothelial growth factor A in bone marrow.Stem Cells. 2007 Aug;25(8):1954-65. doi: 10.1634/stemcells.2006-0688. Epub 2007 May 3. Stem Cells. 2007. PMID: 17478585
-
Monocyte-derived macrophages matured under prolonged hypoxia transcriptionally up-regulate HIF-1α mRNA.Immunobiology. 2011 Jul;216(7):832-9. doi: 10.1016/j.imbio.2010.12.005. Epub 2010 Dec 22. Immunobiology. 2011. PMID: 21281980
-
Does endogenous fatty acid metabolism allow cancer cells to sense hypoxia and mediate hypoxic vasodilatation? Characterization of a novel molecular connection between fatty acid synthase (FAS) and hypoxia-inducible factor-1alpha (HIF-1alpha)-related expression of vascular endothelial growth factor (VEGF) in cancer cells overexpressing her-2/neu oncogene.J Cell Biochem. 2005 Apr 1;94(5):857-63. doi: 10.1002/jcb.20367. J Cell Biochem. 2005. PMID: 15669079
-
Monocytes and dendritic cells in a hypoxic environment: Spotlights on chemotaxis and migration.Immunobiology. 2008;213(9-10):733-49. doi: 10.1016/j.imbio.2008.07.031. Epub 2008 Sep 21. Immunobiology. 2008. PMID: 18926289 Review.
-
Regulation of transendothelial migration of hematopoietic progenitor cells.Ann N Y Acad Sci. 1999 Apr 30;872:176-85; discussion 185-6. doi: 10.1111/j.1749-6632.1999.tb08463.x. Ann N Y Acad Sci. 1999. PMID: 10372121 Review.
Cited by
-
HIF-1α is an important regulator of IL-8 expression in human bone marrow stromal cells under hypoxic microenvironment.Protoplasma. 2024 May;261(3):543-551. doi: 10.1007/s00709-023-01920-z. Epub 2023 Dec 23. Protoplasma. 2024. PMID: 38135806
-
Anoxia Rapidly Induces Changes in Expression of a Large and Diverse Set of Genes in Endothelial Cells.Int J Mol Sci. 2023 Mar 8;24(6):5157. doi: 10.3390/ijms24065157. Int J Mol Sci. 2023. PMID: 36982232 Free PMC article.
-
Extent of Tissue Washing Can Significantly Alter the Composition of Adipose-Derived Stromal Vascular Fraction Cell Preparations: Implications for Clinical Translation.Stem Cells Transl Med. 2023 Jun 15;12(6):391-399. doi: 10.1093/stcltm/szad025. Stem Cells Transl Med. 2023. PMID: 37317551 Free PMC article.
-
Human monocytes respond to lipopolysaccharide (LPS) stimulation in a sex-dependent manner.J Cell Physiol. 2022 Jan;237(1):580-588. doi: 10.1002/jcp.30503. Epub 2021 Jul 12. J Cell Physiol. 2022. PMID: 34252202 Free PMC article.
-
How oxygenation shapes immune responses: emerging roles for physioxia and pathological hypoxia.Nat Rev Immunol. 2025 Mar;25(3):161-177. doi: 10.1038/s41577-024-01087-5. Epub 2024 Sep 30. Nat Rev Immunol. 2025. PMID: 39349943 Review.
References
-
- Antonelli A., Szwargulski P., Scarpa E. S., Thieben F., Cordula G., Ambrosi G., et al. . (2020). Development of long circulating magnetic particle imaging tracers: use of novel magnetic nanoparticles and entrapment into human erythrocytes. Nanomedicine 15, 739–753. 10.2217/nnm-2019-0449, PMID: - DOI - PubMed
-
- Bauer I., Grozio A., Lasigliè D., Basile G., Sturla L., Magnone M., et al. . (2012). The NAD+-dependent histone deacetylase SIRT6 promotes cytokine production and migration in pancreatic cancer cells by regulating Ca2+ responses. J. Biol. Chem. 288, 40924–40937. 10.1074/jbc.M112.405837, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

