The nucleocapsid protein of rice stripe virus in cell nuclei of vector insect regulates viral replication
- PMID: 33675514
- PMCID: PMC7936609
- DOI: 10.1007/s13238-021-00822-1
The nucleocapsid protein of rice stripe virus in cell nuclei of vector insect regulates viral replication
Erratum in
-
Correction to: The nucleocapsid protein of rice stripe virus in cell nuclei of vector insect regulates viral replication.Protein Cell. 2021 Dec;12(12):971-975. doi: 10.1007/s13238-021-00854-7. Protein Cell. 2021. PMID: 34468964 Free PMC article. No abstract available.
Abstract
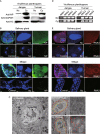
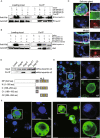
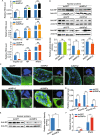
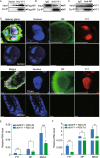
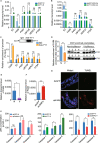
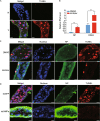
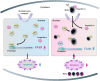
Rice stripe virus (RSV) transmitted by the small brown planthopper causes severe rice yield losses in Asian countries. Although viral nuclear entry promotes viral replication in host cells, whether this phenomenon occurs in vector cells remains unknown. Therefore, in this study, we systematically evaluated the presence and roles of RSV in the nuclei of vector insect cells. We observed that the nucleocapsid protein (NP) and viral genomic RNAs were partially transported into vector cell nuclei by utilizing the importin α nuclear transport system. When blocking NP nuclear localization, cytoplasmic RSV accumulation significantly increased. In the vector cell nuclei, NP bound the transcription factor YY1 and affected its positive regulation to FAIM. Subsequently, decreased FAIM expression triggered an antiviral caspase-dependent apoptotic reaction. Our results reveal that viral nuclear entry induces completely different immune effects in vector and host cells, providing new insights into the balance between viral load and the immunity pressure in vector insects.
Keywords: YY1; importin α; nuclear localization; nucleocapsid protein; rice stripe virus.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Flotillin 2 Facilitates the Infection of a Plant Virus in the Gut of Insect Vector.J Virol. 2022 Apr 13;96(7):e0214021. doi: 10.1128/jvi.02140-21. Epub 2022 Mar 7. J Virol. 2022. PMID: 35254088 Free PMC article.
-
Ribosomal protein L18 is an essential factor that promote rice stripe virus accumulation in small brown planthopper.Virus Res. 2018 Mar 2;247:15-20. doi: 10.1016/j.virusres.2018.01.011. Epub 2018 Jan 31. Virus Res. 2018. PMID: 29374519
-
Rice stripe virus-derived siRNAs play different regulatory roles in rice and in the insect vector Laodelphax striatellus.BMC Plant Biol. 2018 Oct 4;18(1):219. doi: 10.1186/s12870-018-1438-7. BMC Plant Biol. 2018. PMID: 30286719 Free PMC article.
-
The small brown planthopper (Laodelphax striatellus) as a vector of the rice stripe virus.Arch Insect Biochem Physiol. 2023 Feb;112(2):e21992. doi: 10.1002/arch.21992. Epub 2022 Dec 27. Arch Insect Biochem Physiol. 2023. PMID: 36575628 Review.
-
Complex interactions between insect-borne rice viruses and their vectors.Curr Opin Virol. 2018 Dec;33:18-23. doi: 10.1016/j.coviro.2018.07.005. Epub 2018 Jul 19. Curr Opin Virol. 2018. PMID: 30031984 Review.
Cited by
-
Flotillin 2 Facilitates the Infection of a Plant Virus in the Gut of Insect Vector.J Virol. 2022 Apr 13;96(7):e0214021. doi: 10.1128/jvi.02140-21. Epub 2022 Mar 7. J Virol. 2022. PMID: 35254088 Free PMC article.
-
Alternative Splicing Landscape of Small Brown Planthopper and Different Response of JNK2 Isoforms to Rice Stripe Virus Infection.J Virol. 2022 Jan 26;96(2):e0171521. doi: 10.1128/JVI.01715-21. Epub 2021 Nov 10. J Virol. 2022. PMID: 34757837 Free PMC article.
-
Membrane association of importin α facilitates viral entry into salivary gland cells of vector insects.Proc Natl Acad Sci U S A. 2021 Jul 27;118(30):e2103393118. doi: 10.1073/pnas.2103393118. Proc Natl Acad Sci U S A. 2021. PMID: 34290144 Free PMC article.
-
Maintenance of persistent transmission of a plant arbovirus in its insect vector mediated by the Toll-Dorsal immune pathway.Proc Natl Acad Sci U S A. 2024 Apr 2;121(14):e2315982121. doi: 10.1073/pnas.2315982121. Epub 2024 Mar 27. Proc Natl Acad Sci U S A. 2024. PMID: 38536757 Free PMC article.
-
Nucleo-Cytoplasmic Transport of ZIKV Non-Structural 3 Protein Is Mediated by Importin-α/β and Exportin CRM-1.J Virol. 2023 Jan 31;97(1):e0177322. doi: 10.1128/jvi.01773-22. Epub 2022 Dec 8. J Virol. 2023. PMID: 36475764 Free PMC article.
References
-
- Austen M, Luscher B, Luscher-Firzlaff JM. Characterization of the transcriptional regulator YY1. The bipartite transactivation domain is independent of interaction with the TATA box-binding protein, transcription factor IIB, TAFII55, or cAMP-responsive element-binding protein (CPB)-binding protein. J Biol Chem. 1997;272:1709–1717. doi: 10.1074/jbc.272.3.1709. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

