Tissue-Specific Knockdown of Genes of the Argonaute Family Modulates Lifespan and Radioresistance in Drosophila Melanogaster
- PMID: 33673647
- PMCID: PMC7957547
- DOI: 10.3390/ijms22052396
Tissue-Specific Knockdown of Genes of the Argonaute Family Modulates Lifespan and Radioresistance in Drosophila Melanogaster
Abstract
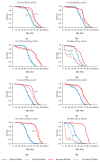
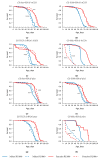
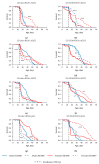
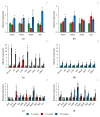
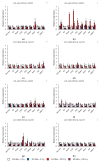
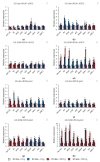
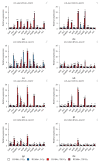
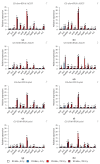
Small RNAs are essential to coordinate many cellular processes, including the regulation of gene expression patterns, the prevention of genomic instability, and the suppression of the mutagenic transposon activity. These processes determine the aging, longevity, and sensitivity of cells and an organism to stress factors (particularly, ionizing radiation). The biogenesis and activity of small RNAs are provided by proteins of the Argonaute family. These proteins participate in the processing of small RNA precursors and the formation of an RNA-induced silencing complex. However, the role of Argonaute proteins in regulating lifespan and radioresistance remains poorly explored. We studied the effect of knockdown of Argonaute genes (AGO1, AGO2, AGO3, piwi) in various tissues on the Drosophila melanogaster lifespan and survival after the γ-irradiation at a dose of 700 Gy. In most cases, these parameters are reduced or did not change significantly in flies with tissue-specific RNA interference. Surprisingly, piwi knockdown in both the fat body and the nervous system causes a lifespan increase. But changes in radioresistance depend on the tissue in which the gene was knocked out. In addition, analysis of changes in retrotransposon levels and expression of stress response genes allow us to determine associated molecular mechanisms.
Keywords: Agronaute; Drosophila melanogaster; aging; ionizing radiation; lifespan; piwi; radioresistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Interaction effect of mutations in the genes (piwi and aub) of the Argonaute family and hobo transposons on the integral survival parameters of Drosophila melanogaster.Biogerontology. 2024 Feb;25(1):131-146. doi: 10.1007/s10522-023-10062-x. Epub 2023 Oct 21. Biogerontology. 2024. PMID: 37864608
-
Multiple roles for Piwi in silencing Drosophila transposons.Genes Dev. 2013 Feb 15;27(4):400-12. doi: 10.1101/gad.209767.112. Epub 2013 Feb 7. Genes Dev. 2013. PMID: 23392609 Free PMC article.
-
The interplay between the Argonaute proteins Piwi and Aub within Drosophila germarium is critical for oogenesis, piRNA biogenesis and TE silencing.Nucleic Acids Res. 2018 Nov 2;46(19):10052-10065. doi: 10.1093/nar/gky695. Nucleic Acids Res. 2018. PMID: 30113668 Free PMC article.
-
Untangling the web: the diverse functions of the PIWI/piRNA pathway.Mol Reprod Dev. 2013 Aug;80(8):632-64. doi: 10.1002/mrd.22195. Epub 2013 Jun 27. Mol Reprod Dev. 2013. PMID: 23712694 Free PMC article. Review.
-
Assembly and function of small RNA - argonaute protein complexes.Biol Chem. 2014 Jun;395(6):611-29. doi: 10.1515/hsz-2014-0116. Biol Chem. 2014. PMID: 24603840 Review.
Cited by
-
Sensory perception of rivals has trait-dependent effects on plasticity in Drosophila melanogaster.Behav Ecol. 2024 Apr 24;35(3):arae031. doi: 10.1093/beheco/arae031. eCollection 2024 May-Jun. Behav Ecol. 2024. PMID: 38680228 Free PMC article.
-
Exposure to Temperature and Insecticides Modulates the Expression of Small Noncoding RNA-Associated Transcripts in the Colorado Potato Beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae).J Insect Sci. 2022 Jan 1;22(1):23. doi: 10.1093/jisesa/ieac004. J Insect Sci. 2022. PMID: 35172010 Free PMC article.
-
Interaction effect of mutations in the genes (piwi and aub) of the Argonaute family and hobo transposons on the integral survival parameters of Drosophila melanogaster.Biogerontology. 2024 Feb;25(1):131-146. doi: 10.1007/s10522-023-10062-x. Epub 2023 Oct 21. Biogerontology. 2024. PMID: 37864608
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

