Human Astroviruses: A Tale of Two Strains
- PMID: 33673521
- PMCID: PMC7997325
- DOI: 10.3390/v13030376
Human Astroviruses: A Tale of Two Strains
Abstract
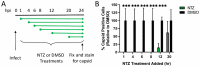
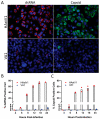
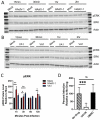
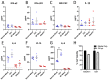
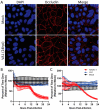
Since the 1970s, eight closely related serotypes of classical human astroviruses (HAstV) have been associated with gastrointestinal illness worldwide. In the late 2000s, three genetically unique human astrovirus clades, VA1-VA3, VA2-VA4, and MLB, were described. While the exact disease associated with these clades remains to be defined, VA1 has been associated with central nervous system infections. The discovery that VA1 could be grown in cell culture, supports exciting new studies aimed at understanding viral pathogenesis. Given the association of VA1 with often lethal CNS infections, we tested its susceptibility to the antimicrobial drug, nitazoxanide (NTZ), which we showed could inhibit classical HAstV infections. Our studies demonstrate that NTZ inhibited VA1 replication in Caco2 cells even when added at 12 h post-infection, which is later than in HAstV-1 infection. These data led us to further probe VA1 replication kinetics and cellular responses to infection in Caco-2 cells in comparison to the well-studied HAstV-1 strain. Overall, our studies highlight that VA1 replicates more slowly than HAstV-1 and elicits significantly different cellular responses, including the inability to disrupt cellular junctions and barrier permeability.
Keywords: HAstV-1; VA1; barrier permeability; human astrovirus; nitazoxanide; viral replication.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Astrovirus infection in hospitalized children: Molecular, clinical and epidemiological features.J Clin Virol. 2017 Sep;94:79-85. doi: 10.1016/j.jcv.2017.07.014. Epub 2017 Jul 25. J Clin Virol. 2017. PMID: 28772169
-
Mouse and human immune responses share neutralization epitopes of HAstV-VA1.J Virol. 2024 Jul 23;98(7):e0097124. doi: 10.1128/jvi.00971-24. Epub 2024 Jun 25. J Virol. 2024. PMID: 38916399 Free PMC article.
-
Infection and Propagation of Astrovirus VA1 in Cell Culture.Curr Protoc Microbiol. 2019 Feb;52(1):e73. doi: 10.1002/cpmc.73. Epub 2018 Nov 16. Curr Protoc Microbiol. 2019. PMID: 30444308 Free PMC article.
-
Novel human astroviruses: Novel human diseases?J Clin Virol. 2016 Sep;82:56-63. doi: 10.1016/j.jcv.2016.07.004. Epub 2016 Jul 11. J Clin Virol. 2016. PMID: 27434149 Review.
-
Astrovirus Pathogenesis.Viruses. 2017 Jan 22;9(1):22. doi: 10.3390/v9010022. Viruses. 2017. PMID: 28117758 Free PMC article. Review.
Cited by
-
Neurotropic Astroviruses in Animals.Viruses. 2021 Jun 23;13(7):1201. doi: 10.3390/v13071201. Viruses. 2021. PMID: 34201545 Free PMC article. Review.
-
Structure and antigenicity of the divergent human astrovirus VA1 capsid spike.PLoS Pathog. 2024 Feb 28;20(2):e1012028. doi: 10.1371/journal.ppat.1012028. eCollection 2024 Feb. PLoS Pathog. 2024. PMID: 38416796 Free PMC article.
-
High Seropositivity Rate of Neutralizing Antibodies to Astrovirus VA1 in Human Populations.mSphere. 2021 Oct 27;6(5):e0048421. doi: 10.1128/mSphere.00484-21. Epub 2021 Sep 1. mSphere. 2021. PMID: 34468168 Free PMC article.
-
Astrovirus replication is dependent on induction of double-membrane vesicles through a PI3K-dependent, LC3-independent pathway.J Virol. 2023 Sep 28;97(9):e0102523. doi: 10.1128/jvi.01025-23. Epub 2023 Sep 5. J Virol. 2023. PMID: 37668367 Free PMC article.
-
Beyond the Gastrointestinal Tract: The Emerging and Diverse Tissue Tropisms of Astroviruses.Viruses. 2021 Apr 22;13(5):732. doi: 10.3390/v13050732. Viruses. 2021. PMID: 33922259 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

