Association of Adipose Tissue and Adipokines with Development of Obesity-Induced Liver Cancer
- PMID: 33671547
- PMCID: PMC7926723
- DOI: 10.3390/ijms22042163
Association of Adipose Tissue and Adipokines with Development of Obesity-Induced Liver Cancer
Abstract
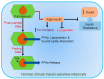
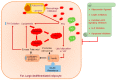
Obesity is rapidly dispersing all around the world and is closely associated with a high risk of metabolic diseases such as insulin resistance, dyslipidemia, and nonalcoholic fatty liver disease (NAFLD), leading to carcinogenesis, especially hepatocellular carcinoma (HCC). It results from an imbalance between food intake and energy expenditure, leading to an excessive accumulation of adipose tissue (AT). Adipocytes play a substantial role in the tumor microenvironment through the secretion of several adipokines, affecting cancer progression, metastasis, and chemoresistance via diverse signaling pathways. AT is considered an endocrine organ owing to its ability to secrete adipokines, such as leptin, adiponectin, resistin, and a plethora of inflammatory cytokines, which modulate insulin sensitivity and trigger chronic low-grade inflammation in different organs. Even though the precise mechanisms are still unfolding, it is now established that the dysregulated secretion of adipokines by AT contributes to the development of obesity-related metabolic disorders. This review focuses on several obesity-associated adipokines and their impact on obesity-related metabolic diseases, subsequent metabolic complications, and progression to HCC, as well as their role as potential therapeutic targets. The field is rapidly developing, and further research is still required to fully understand the underlying mechanisms for the metabolic actions of adipokines and their role in obesity-associated HCC.
Keywords: HCC; NAFLD/NASH; adipokines; adiponectin; adipose tissue; leptin; therapeutic targets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Obesity, adipokines and hepatocellular carcinoma.Int J Cancer. 2013 Oct 15;133(8):1776-83. doi: 10.1002/ijc.28105. Epub 2013 Mar 7. Int J Cancer. 2013. PMID: 23404222 Review.
-
Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease.Int J Mol Sci. 2014 Apr 11;15(4):6184-223. doi: 10.3390/ijms15046184. Int J Mol Sci. 2014. PMID: 24733068 Free PMC article. Review.
-
Adipokines and Non-Alcoholic Fatty Liver Disease: Multiple Interactions.Int J Mol Sci. 2017 Jul 29;18(8):1649. doi: 10.3390/ijms18081649. Int J Mol Sci. 2017. PMID: 28758929 Free PMC article. Review.
-
Relevance of Leptin and Other Adipokines in Obesity-Associated Cardiovascular Risk.Nutrients. 2019 Nov 5;11(11):2664. doi: 10.3390/nu11112664. Nutrients. 2019. PMID: 31694146 Free PMC article. Review.
-
The Role of Adipose Tissue and Adipokines in Sepsis: Inflammatory and Metabolic Considerations, and the Obesity Paradox.Curr Obes Rep. 2019 Dec;8(4):434-457. doi: 10.1007/s13679-019-00360-2. Curr Obes Rep. 2019. PMID: 31637623 Review.
Cited by
-
Biomarkers of Oncogenesis, Adipose Tissue Dysfunction and Systemic Inflammation for the Detection of Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease.Cancers (Basel). 2021 May 11;13(10):2305. doi: 10.3390/cancers13102305. Cancers (Basel). 2021. PMID: 34064999 Free PMC article.
-
The Role of the Adipokine Resistin in the Pathogenesis and Progression of Epithelial Ovarian Cancer.Biomedicines. 2022 Apr 16;10(4):920. doi: 10.3390/biomedicines10040920. Biomedicines. 2022. PMID: 35453670 Free PMC article. Review.
-
Do low skeletal muscle bulk and disturbed body fat mass impact tumor recurrence in stage I/II hepatocellular carcinoma undergoing surgery? An observational cohort study.Int J Surg. 2024 Nov 1;110(11):7067-7079. doi: 10.1097/JS9.0000000000001905. Int J Surg. 2024. PMID: 38959093 Free PMC article.
-
Increased Expression Levels of Netrin-1 in Visceral Adipose Tissue during Obesity Favour Colon Cancer Cell Migration.Cancers (Basel). 2023 Feb 7;15(4):1038. doi: 10.3390/cancers15041038. Cancers (Basel). 2023. PMID: 36831381 Free PMC article.
-
Bifidobacterium bifidum DS0908 and Bifidobacterium longum DS0950 Culture-Supernatants Ameliorate Obesity-Related Characteristics in Mice with High-Fat Diet-Induced Obesity.J Microbiol Biotechnol. 2023 Jan 28;33(1):96-105. doi: 10.4014/jmb.2210.10046. Epub 2022 Nov 22. J Microbiol Biotechnol. 2023. PMID: 36457182 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

