Generation of Liposomes to Study the Effect of Mycobacterium Tuberculosis Lipids on HIV-1 cis- and trans-Infections
- PMID: 33669411
- PMCID: PMC7920488
- DOI: 10.3390/ijms22041945
Generation of Liposomes to Study the Effect of Mycobacterium Tuberculosis Lipids on HIV-1 cis- and trans-Infections
Abstract
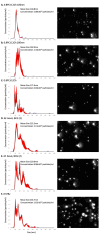
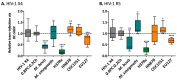
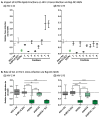
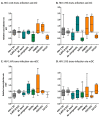
Tuberculosis (TB) is the leading cause of death among HIV-1-infected individuals and Mycobacterium tuberculosis (Mtb) co-infection is an early precipitate to AIDS. We aimed to determine whether Mtb strains differentially modulate cellular susceptibility to HIV-1 infection (cis- and trans-infection), via surface receptor interaction by their cell envelope lipids. Total lipids from pathogenic (lineage 4 Mtb H37Rv, CDC1551 and lineage 2 Mtb HN878, EU127) and non-pathogenic (Mycobacterium bovis BCG and Mycobacterium smegmatis) Mycobacterium strains were integrated into liposomes mimicking the lipid distribution and antigen accessibility of the mycobacterial cell wall. The resulting liposomes were tested for modulating in vitro HIV-1 cis- and trans-infection of TZM-bl cells using single-cycle infectious virus particles. Mtb glycolipids did not affect HIV-1 direct infection however, trans-infection of both R5 and X4 tropic HIV-1 strains were impaired in the presence of glycolipids from M. bovis, Mtb H37Rv and Mtb EU127 strains when using Raji-DC-SIGN cells or immature and mature dendritic cells (DCs) to capture virus. SL1, PDIM and TDM lipids were identified to be involved in DC-SIGN recognition and impairment of HIV-1 trans-infection. These findings indicate that variant strains of Mtb have differential effect on HIV-1 trans-infection with the potential to influence HIV-1 disease course in co-infected individuals.
Keywords: BCG; CDC1551; DC-SIGN; EU127; H37Rv; HIV-1; HN878; M. smegmatis; Mycobacterium tuberculosis; PDIM; SL1; TB; TDM; in vitro; liposomes; trans-infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Mycobacteria target DC-SIGN to suppress dendritic cell function.J Exp Med. 2003 Jan 6;197(1):7-17. doi: 10.1084/jem.20021229. J Exp Med. 2003. PMID: 12515809 Free PMC article.
-
Mycobacterium tuberculosis complex enhances susceptibility of CD4 T cells to HIV through a TLR2-mediated pathway.PLoS One. 2012;7(7):e41093. doi: 10.1371/journal.pone.0041093. Epub 2012 Jul 23. PLoS One. 2012. PMID: 22844428 Free PMC article.
-
Divergent effects of mycobacterial cell wall glycolipids on maturation and function of human monocyte-derived dendritic cells.PLoS One. 2012;7(8):e42515. doi: 10.1371/journal.pone.0042515. Epub 2012 Aug 3. PLoS One. 2012. PMID: 22880012 Free PMC article.
-
DC-SIGN and mannosylated surface structures of Mycobacterium tuberculosis: a deceptive liaison.Eur J Cell Biol. 2010 Jan;89(1):95-101. doi: 10.1016/j.ejcb.2009.10.004. Epub 2009 Nov 4. Eur J Cell Biol. 2010. PMID: 19892432 Review.
-
Lipoarabinomannan, and its related glycolipids, induce divergent and opposing immune responses to Mycobacterium tuberculosis depending on structural diversity and experimental variations.Tuberculosis (Edinb). 2016 Jan;96:120-30. doi: 10.1016/j.tube.2015.09.005. Epub 2015 Oct 28. Tuberculosis (Edinb). 2016. PMID: 26586646 Review.
Cited by
-
A Quantitative Method for the Study of HIV-1 and Mycobacterium tuberculosis Coinfection.J Infect Dis. 2023 Mar 1;227(5):708-713. doi: 10.1093/infdis/jiac491. J Infect Dis. 2023. PMID: 36537213 Free PMC article.
-
Glycan dependent phenotype differences of HIV-1 generated from macrophage versus CD4+ T helper cell populations.Front Immunol. 2023 Jun 21;14:1107349. doi: 10.3389/fimmu.2023.1107349. eCollection 2023. Front Immunol. 2023. PMID: 37415979 Free PMC article.
-
Systems biology approaches to investigate the role of granulomas in TB-HIV coinfection.Front Immunol. 2022 Oct 31;13:1014515. doi: 10.3389/fimmu.2022.1014515. eCollection 2022. Front Immunol. 2022. PMID: 36405707 Free PMC article. Review.
References
-
- World Health Organization Global Tuberculosis Report. WHO; Geneva, Switzerland: 2020.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

