Examining the Relationship between Circulating CD4- CD8- Double-Negative T Cells and Outcomes of Immuno-Checkpoint Inhibitor Therapy-Looking for Biomarkers and Therapeutic Targets in Metastatic Melanoma
- PMID: 33669266
- PMCID: PMC7920027
- DOI: 10.3390/cells10020406
Examining the Relationship between Circulating CD4- CD8- Double-Negative T Cells and Outcomes of Immuno-Checkpoint Inhibitor Therapy-Looking for Biomarkers and Therapeutic Targets in Metastatic Melanoma
Abstract
Background: The role of circulating CD4-/CD8- double-negative T cells (DNTs) in the immune response to melanoma is poorly understood, as are the effects of checkpoint inhibitors on T cell subpopulations.
Methods: We performed a basal and longitudinal assessment of circulating immune cells, including DNTs, in metastatic melanoma patients treated with checkpoint blockade in a single-center cohort, and examined the correlations levels of immune cells with clinical features and therapy outcomes.
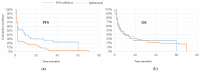
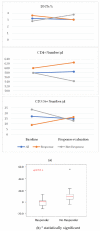
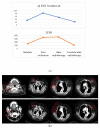
Results: Sixty-eight patients (48 ipilimumab, 20 PD1 inhibitors) were enrolled in the study. Our analysis indicated that better outcomes were associated with normal LDH, fewer than three metastatic sites, an ECOG performance status of 0, M1a stage, lower WBC and a higher lymphocyte count. The increase in lymphocyte count and decrease of DNTs were significantly associated with the achievement of an overall response. The median value of DNT decreased while the CD4+ and NK cells increased in patients that responded to treatment compare to those who did not respond to treatment.
Conclusions: DNT cells change during treatment with checkpoint inhibitors and may be adept at sensing the immune response to melanoma. The complementary variation of DNT cells with respect to CD4+ and other immune actors may improve the reliability of lymphocyte assessment. Further investigation of DNT as a potential target in checkpoint inhibitor resistant melanoma is warranted.
Keywords: checkpoint inhibitors; double negative T cells; immunotherapy resistance; melanoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The role of tumor-infiltrating lymphocytes (TILs) as a predictive biomarker of response to anti-PD1 therapy in patients with metastatic non-small cell lung cancer or metastatic melanoma.Med Oncol. 2018 Jan 31;35(3):25. doi: 10.1007/s12032-018-1080-0. Med Oncol. 2018. PMID: 29388007
-
Prognostic factors and outcomes in metastatic uveal melanoma treated with programmed cell death-1 or combined PD-1/cytotoxic T-lymphocyte antigen-4 inhibition.Eur J Cancer. 2017 Sep;82:56-65. doi: 10.1016/j.ejca.2017.05.038. Epub 2017 Jun 22. Eur J Cancer. 2017. PMID: 28648699
-
Association of lymphocyte subsets with efficacy and prognosis of immune checkpoint inhibitor therapy in advanced non-small cell lung carcinoma: a retrospective study.BMC Pulm Med. 2022 Apr 28;22(1):166. doi: 10.1186/s12890-022-01951-x. BMC Pulm Med. 2022. PMID: 35484541 Free PMC article.
-
Immune modulations during chemoimmunotherapy & novel vaccine strategies--in metastatic melanoma and non small-cell lung cancer.Dan Med J. 2013 Dec;60(12):B4774. Dan Med J. 2013. PMID: 24355457 Review.
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
Cited by
-
Lymphocyte T Subsets and Outcome of Immune Checkpoint Inhibitors in Melanoma Patients: An Oncologist's Perspective on Current Knowledge.Int J Mol Sci. 2024 Aug 31;25(17):9506. doi: 10.3390/ijms25179506. Int J Mol Sci. 2024. PMID: 39273452 Free PMC article. Review.
-
Characterization of double-negative T cells in colorectal cancers and their corresponding lymph nodes.Oncoimmunology. 2024 Jul 4;13(1):2373530. doi: 10.1080/2162402X.2024.2373530. eCollection 2024. Oncoimmunology. 2024. PMID: 38979545 Free PMC article.
-
Thyroid Cancer Screening Using Tumor-Associated DN T Cells as Immunogenomic Markers.Front Oncol. 2022 May 27;12:891002. doi: 10.3389/fonc.2022.891002. eCollection 2022. Front Oncol. 2022. PMID: 35692772 Free PMC article.
-
Double-negative T cells: a promising avenue of adoptive cell therapy in transplant oncology.J Zhejiang Univ Sci B. 2023 May 15;24(5):387-396. doi: 10.1631/jzus.B2200528. J Zhejiang Univ Sci B. 2023. PMID: 37190888 Free PMC article.
-
Circulating T cells: a promising biomarker of anti-PD-(L)1 therapy.Front Immunol. 2024 Mar 21;15:1371559. doi: 10.3389/fimmu.2024.1371559. eCollection 2024. Front Immunol. 2024. PMID: 38576625 Free PMC article. Review.
References
-
- Simeone E., Gentilcore G., Giannarelli D., Grimaldi A.M., Caracò C., Curvietto M., Esposito A., Paone M., Palla M., Cavalcanti E., et al. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol. Immunother. 2014;63:675–683. doi: 10.1007/s00262-014-1545-8. - DOI - PMC - PubMed
-
- Martens A., Wistuba-Hamprecht K., Yuan J., Postow M.A., Wong P., Capone M., Madonna G., Khammari A., Schilling B., Sucker A., et al. Increases in Absolute Lymphocytes and Circulating CD4+ and CD8+ T Cells Are Associated with Positive Clinical Outcome of Melanoma Patients Treated with Ipilimumab. Clin. Cancer Res. 2016;22:4848–4858. doi: 10.1158/1078-0432.CCR-16-0249. - DOI - PMC - PubMed
-
- Weide B., Martens A., Hassel J.C., Berking C., Postow M.A., Bisschop K., Simeone E., Mangana J., Schilling B., Di Giacomo A.M., et al. Baseline Biomarkers for Outcome of Melanoma Patients Treated with Pembrolizumab. Clin. Cancer Res. 2016;22:5487–5496. doi: 10.1158/1078-0432.CCR-16-0127. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

