Syndecans and Pancreatic Ductal Adenocarcinoma
- PMID: 33669066
- PMCID: PMC7996579
- DOI: 10.3390/biom11030349
Syndecans and Pancreatic Ductal Adenocarcinoma
Abstract
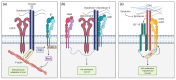
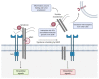
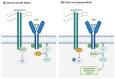
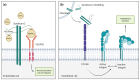
Pancreatic Ductal Adenocarcinoma (PDAC) is a fatal disease with poor prognosis because patients rarely express symptoms in initial stages, which prevents early detection and diagnosis. Syndecans, a subfamily of proteoglycans, are involved in many physiological processes including cell proliferation, adhesion, and migration. Syndecans are physiologically found in many cell types and their interactions with other macromolecules enhance many pathways. In particular, extracellular matrix components, growth factors, and integrins collect the majority of syndecans associations acting as biochemical, physical, and mechanical transducers. Syndecans are transmembrane glycoproteins, but occasionally their extracellular domain can be released from the cell surface by the action of matrix metalloproteinases, converting them into soluble molecules that are capable of binding distant molecules such as extracellular matrix (ECM) components, growth factor receptors, and integrins from other cells. In this review, we explore the role of syndecans in tumorigenesis as well as their potential as therapeutic targets. Finally, this work reviews the contribution of syndecan-1 and syndecan-2 in PDAC progression and illustrates its potential to be targeted in future treatments for this devastating disease.
Keywords: angiogenesis; pancreatic ductal adenocarcinoma; proteoglycans; syndecans; tumor progression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Syndecans in heart fibrosis.Cell Tissue Res. 2016 Sep;365(3):539-52. doi: 10.1007/s00441-016-2454-2. Epub 2016 Jul 14. Cell Tissue Res. 2016. PMID: 27411689 Review.
-
Syndecans shed their reputation as inert molecules.Sci Signal. 2009 Mar 31;2(64):pe18. doi: 10.1126/scisignal.264pe18. Sci Signal. 2009. PMID: 19336838 Free PMC article. Review.
-
Syndecans - key regulators of cell signaling and biological functions.FEBS J. 2017 Jan;284(1):27-41. doi: 10.1111/febs.13940. Epub 2016 Nov 18. FEBS J. 2017. PMID: 27790852 Review.
-
Syndecans as receptors and organizers of the extracellular matrix.Cell Tissue Res. 2010 Jan;339(1):31-46. doi: 10.1007/s00441-009-0829-3. Epub 2009 Jul 14. Cell Tissue Res. 2010. PMID: 19597846 Review.
-
Proteoglycans, ion channels and cell-matrix adhesion.Biochem J. 2017 May 25;474(12):1965-1979. doi: 10.1042/BCJ20160747. Biochem J. 2017. PMID: 28546458 Review.
Cited by
-
Identification and Validation of Three PDAC Subtypes and Individualized GSVA Immune Pathway-Related Prognostic Risk Score Formula in Pancreatic Ductal Adenocarcinoma Patients.J Oncol. 2021 Dec 27;2021:4986227. doi: 10.1155/2021/4986227. eCollection 2021. J Oncol. 2021. PMID: 34987579 Free PMC article.
-
Integrated bioinformatics analysis reveals upregulated extracellular matrix hub genes in pancreatic cancer: Implications for diagnosis, prognosis, immune infiltration, and therapeutic strategies.Cancer Rep (Hoboken). 2024 Apr;7(4):e2059. doi: 10.1002/cnr2.2059. Cancer Rep (Hoboken). 2024. PMID: 38639039 Free PMC article.
-
SDC1 and ITGA2 as novel prognostic biomarkers for PDAC related to IPMN.Sci Rep. 2023 Oct 31;13(1):18727. doi: 10.1038/s41598-023-44646-x. Sci Rep. 2023. PMID: 37907515 Free PMC article.
-
Advanced iron oxide nanotheranostics for multimodal and precision treatment of pancreatic ductal adenocarcinoma.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022 Jul;14(4):e1793. doi: 10.1002/wnan.1793. Epub 2022 Apr 9. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022. PMID: 35396932 Free PMC article. Review.
-
The Role of Periostin in Angiogenesis and Lymphangiogenesis in Tumors.Cancers (Basel). 2022 Aug 30;14(17):4225. doi: 10.3390/cancers14174225. Cancers (Basel). 2022. PMID: 36077762 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

