Why Cells and Viruses Cannot Survive without an ESCRT
- PMID: 33668191
- PMCID: PMC7995964
- DOI: 10.3390/cells10030483
Why Cells and Viruses Cannot Survive without an ESCRT
Abstract
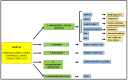
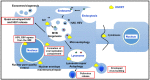
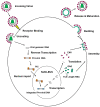
Intracellular organelles enwrapped in membranes along with a complex network of vesicles trafficking in, out and inside the cellular environment are one of the main features of eukaryotic cells. Given their central role in cell life, compartmentalization and mechanisms allowing their maintenance despite continuous crosstalk among different organelles have been deeply investigated over the past years. Here, we review the multiple functions exerted by the endosomal sorting complex required for transport (ESCRT) machinery in driving membrane remodeling and fission, as well as in repairing physiological and pathological membrane damages. In this way, ESCRT machinery enables different fundamental cellular processes, such as cell cytokinesis, biogenesis of organelles and vesicles, maintenance of nuclear-cytoplasmic compartmentalization, endolysosomal activity. Furthermore, we discuss some examples of how viruses, as obligate intracellular parasites, have evolved to hijack the ESCRT machinery or part of it to execute/optimize their replication cycle/infection. A special emphasis is given to the herpes simplex virus type 1 (HSV-1) interaction with the ESCRT proteins, considering the peculiarities of this interplay and the need for HSV-1 to cross both the nuclear-cytoplasmic and the cytoplasmic-extracellular environment compartmentalization to egress from infected cells.
Keywords: ESCRT; HSV-1; cellular membranes; extracellular vesicles; viruses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
[Cellular ESCRT complex and its roles in enveloped viruses budding].Sheng Wu Gong Cheng Xue Bao. 2012 Sep;28(9):1031-7. Sheng Wu Gong Cheng Xue Bao. 2012. PMID: 23289305 Review. Chinese.
-
Herpes simplex virus type 1 production requires a functional ESCRT-III complex but is independent of TSG101 and ALIX expression.J Virol. 2009 Nov;83(21):11254-64. doi: 10.1128/JVI.00574-09. Epub 2009 Aug 19. J Virol. 2009. PMID: 19692479 Free PMC article.
-
[Virus hijacking ESCRT system to promote self-replication: a review].Sheng Wu Gong Cheng Xue Bao. 2023 Oct 25;39(10):3948-3965. doi: 10.13345/j.cjb.230323. Sheng Wu Gong Cheng Xue Bao. 2023. PMID: 37877384 Review. Chinese.
-
ESCRT & Co.Biol Cell. 2010 Mar 12;102(5):293-318. doi: 10.1042/BC20090161. Biol Cell. 2010. PMID: 20222872 Review.
-
The multifaceted interactions between pathogens and host ESCRT machinery.PLoS Pathog. 2023 May 4;19(5):e1011344. doi: 10.1371/journal.ppat.1011344. eCollection 2023 May. PLoS Pathog. 2023. PMID: 37141275 Free PMC article. Review.
Cited by
-
EhVps23: A Component of ESCRT-I That Participates in Vesicular Trafficking and Phagocytosis of Entamoeba histolytica.Front Cell Infect Microbiol. 2021 Oct 29;11:770759. doi: 10.3389/fcimb.2021.770759. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34778112 Free PMC article.
-
Human Milk Extracellular Vesicles: A Biological System with Clinical Implications.Cells. 2022 Jul 30;11(15):2345. doi: 10.3390/cells11152345. Cells. 2022. PMID: 35954189 Free PMC article. Review.
-
The Viral Origin of Human Breast Cancer: From the Mouse Mammary Tumor Virus (MMTV) to the Human Betaretrovirus (HBRV).Viruses. 2022 Aug 1;14(8):1704. doi: 10.3390/v14081704. Viruses. 2022. PMID: 36016325 Free PMC article. Review.
-
Deciphering COVID-19 host transcriptomic complexity and variations for therapeutic discovery against new variants.iScience. 2022 Oct 21;25(10):105068. doi: 10.1016/j.isci.2022.105068. Epub 2022 Sep 3. iScience. 2022. PMID: 36093376 Free PMC article.
-
The Role of Exosome and the ESCRT Pathway on Enveloped Virus Infection.Int J Mol Sci. 2021 Aug 22;22(16):9060. doi: 10.3390/ijms22169060. Int J Mol Sci. 2021. PMID: 34445766 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

