Selective Cardiomyocyte Oxidative Stress Leads to Bystander Senescence of Cardiac Stromal Cells
- PMID: 33668142
- PMCID: PMC7956294
- DOI: 10.3390/ijms22052245
Selective Cardiomyocyte Oxidative Stress Leads to Bystander Senescence of Cardiac Stromal Cells
Abstract
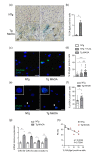
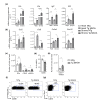
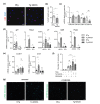
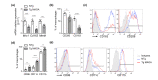
Accumulation of senescent cells in tissues during normal or accelerated aging has been shown to be detrimental and to favor the outcomes of age-related diseases such as heart failure (HF). We have previously shown that oxidative stress dependent on monoamine oxidase A (MAOA) activity in cardiomyocytes promotes mitochondrial damage, the formation of telomere-associated foci, senescence markers, and triggers systolic cardiac dysfunction in a model of transgenic mice overexpressing MAOA in cardiomyocytes (Tg MAOA). However, the impact of cardiomyocyte oxidative stress on the cardiac microenvironment in vivo is still unclear. Our results showed that systolic cardiac dysfunction in Tg MAOA mice was strongly correlated with oxidative stress induced premature senescence of cardiac stromal cells favoring the recruitment of CCR2+ monocytes and the installation of cardiac inflammation. Understanding the interplay between oxidative stress induced premature senescence and accelerated cardiac dysfunction will help to define new molecular pathways at the crossroad between cardiac dysfunction and accelerated aging, which could contribute to the increased susceptibility of the elderly to HF.
Keywords: CCR2+ Macrophages; SASP; cardiac mesenchymal stromal cells; monoamine oxidase A; oxidative stress; stress-induced premature senescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
p53-PGC-1α pathway mediates oxidative mitochondrial damage and cardiomyocyte necrosis induced by monoamine oxidase-A upregulation: role in chronic left ventricular dysfunction in mice.Antioxid Redox Signal. 2013 Jan 1;18(1):5-18. doi: 10.1089/ars.2011.4373. Epub 2012 Aug 10. Antioxid Redox Signal. 2013. PMID: 22738191 Free PMC article.
-
Length-independent telomere damage drives post-mitotic cardiomyocyte senescence.EMBO J. 2019 Mar 1;38(5):e100492. doi: 10.15252/embj.2018100492. Epub 2019 Feb 8. EMBO J. 2019. PMID: 30737259 Free PMC article.
-
Monoamine oxidase-A is a novel driver of stress-induced premature senescence through inhibition of parkin-mediated mitophagy.Aging Cell. 2018 Oct;17(5):e12811. doi: 10.1111/acel.12811. Epub 2018 Jul 12. Aging Cell. 2018. PMID: 30003648 Free PMC article.
-
Cardiomyocyte Senescence and Cellular Communications Within Myocardial Microenvironments.Front Endocrinol (Lausanne). 2020 May 21;11:280. doi: 10.3389/fendo.2020.00280. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32508749 Free PMC article. Review.
-
The role of cardiomyocyte senescence in cardiovascular diseases: A molecular biology update.Eur J Pharmacol. 2024 Nov 15;983:176961. doi: 10.1016/j.ejphar.2024.176961. Epub 2024 Aug 28. Eur J Pharmacol. 2024. PMID: 39209099 Review.
Cited by
-
Clearance of Stress-Induced Premature Senescent Cells Alleviates the Formation of Abdominal Aortic Aneurysms.Aging Dis. 2023 Oct 1;14(5):1778-1798. doi: 10.14336/AD.2023.0215. Aging Dis. 2023. PMID: 37196124 Free PMC article.
-
Importance of Mitochondria in Cardiac Pathologies: Focus on Uncoupling Proteins and Monoamine Oxidases.Int J Mol Sci. 2023 Mar 30;24(7):6459. doi: 10.3390/ijms24076459. Int J Mol Sci. 2023. PMID: 37047436 Free PMC article. Review.
-
Aging and aging-related diseases: from molecular mechanisms to interventions and treatments.Signal Transduct Target Ther. 2022 Dec 16;7(1):391. doi: 10.1038/s41392-022-01251-0. Signal Transduct Target Ther. 2022. PMID: 36522308 Free PMC article. Review.
-
Identification of Prominin-2 as a new player of cardiomyocyte senescence in the aging heart.Aging Cell. 2024 Sep;23(9):e14204. doi: 10.1111/acel.14204. Epub 2024 May 17. Aging Cell. 2024. PMID: 38757782 Free PMC article.
-
Therapeutic opportunities for senolysis in cardiovascular disease.FEBS J. 2023 Mar;290(5):1235-1255. doi: 10.1111/febs.16351. Epub 2022 Jan 25. FEBS J. 2023. PMID: 35015342 Free PMC article. Review.
References
-
- Dai D.F., Santana L.F., Vermulst M., Tomazela D.M., Emond M.J., MacCoss M.J., Gollahon K., Martin G.M., Loeb L.A., Ladiges W.C., et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

